Spinal Cord Implant Allows Paraplegics to Walk Again, Scientists Say
Feb. 7, 2022
Three men paralyzed with severe spinal cord injuries were able to walk again days after receiving a spinal cord implant that stimulates trunk and leg muscles — a development scientists think could have broad application as a commercial product.
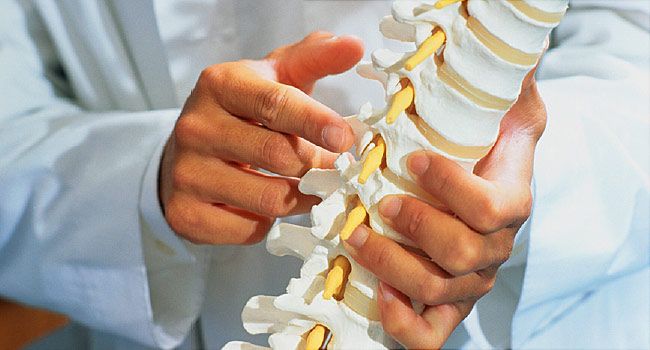
Scientists implanted 16-electrode devices into the epidural space on the men’s spines, between the vertebrae and the spinal cord membrane, CNN reported. The electrodes receive electrical currents from pacemakers implanted under the skin of their abdomens that are wirelessly controlled with a tablet computer, CNN said.
Michel Roccati of Italy, who lost his ability to walk in a 2017 motorcycle accident, said now that he has the implant, he can move around town with a walker and stand to take a shower.
“I am free,” Roccati said. “I can walk wherever I want to.”
The study was led by Jocelyne Bloch from Lausanne University Hospital and Grégoire Courtine of the Swiss Federal Institute of Technology. The results were published Monday in the journal Nature Medicine.
Electrical stimulation of the spine has been studied for years but hasn’t shown such immediate results.
In 2018, for example, the Mayo Clinic in the United States said a man paralyzed in a snowmobile accident was able to walk again with a spinal implant, but only after 22 weeks of physical therapy.
The men in the recent study had lost all voluntary movement below the site of their injuries but were able to take steps on a treadmill the day after surgery, CNN said.
“It’s a very emotional moment, because [patients] realize they can step,” Bloch said.
Physical therapy and three to four months of training was required before the men the Swiss study could complete actions such as climbing stairs or walking 500 meters independently, CNN said.
“For the first time, we have not only immediate effect — though training is still important — but also individuals with no sensation, no movement whatsoever, have been able to regain full standing and walking independently of the laboratory,” Courtine said.
USA Today reported that the Swiss team hopes to begin a 50-100 patient clinical trial within a few years and eventually a 1,000-person trial to gain approval from the U.S. Food and Drug Administration. If approved, the technology could provide new hope for thousands of paralyzed people.
Courtine told USA Today the research team’s next goal is to control the electrodes with a cell phone.
The researchers said the FDA approved a “breakthrough devices” designation for the technology, which would allow people to obtain coverage through the Medicare Coverage of Innovative Technology program, CNN said.