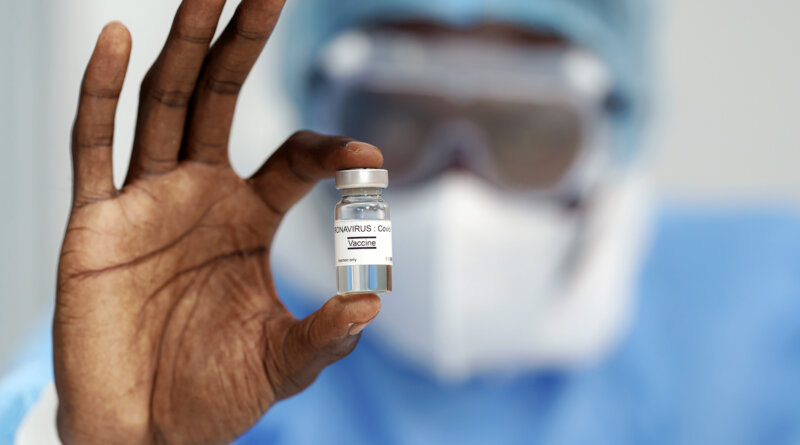
FDA Authorizes Pfizer COVID Vaccine for Kids 12-15
[ad_1]
May 10, 2021 — The FDA on Monday granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children 12-to-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall — a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not quite a done deal.
Next, the CDC’s Advisory Committee on Immunization Practices will decide whether to recommend use of the vaccine in this age group, CNN reported. That group is scheduled to meet Wednesday. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on Monday amends the Dec. 11 emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna has also launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID pandemic can truly be brought under control. The 12-to-15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatricians president Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents,” Beers said in a statement. “Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
Last week President Joe Biden announced a new strategy for expanding vaccinations in which vaccinating 12-to-15-year-old was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12 to 15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bouria, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.”
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts.
“I would like to thank all of the courageous participants, who selflessly raised their hands to join our clinical trial. Without them—and their families and caregivers, this milestone would not be possible.”
[ad_2]
Source link



buy lanoxin 250 mg sale order generic digoxin order molnupiravir 200 mg generic
diamox tablet isosorbide for sale imuran 25mg us
order digoxin 250mg pills purchase micardis online cheap buy molnunat 200mg without prescription
order amoxicillin 250mg for sale stromectol cheap ivermectin 12mg pills
buy carvedilol 25mg for sale buy amitriptyline 50mg generic buy generic elavil 10mg
priligy 60mg usa order generic motilium motilium 10mg tablet
buy fosamax 35mg online cheap order nitrofurantoin for sale order ibuprofen 600mg pill
buy indocin generic indocin over the counter cenforce online buy
buy pamelor generic panadol 500mg without prescription order paxil 20mg without prescription
buy doxycycline 200mg online chloroquine 250mg uk methylprednisolone 16 mg over the counter
pepcid us famotidine order remeron drug
tadalafil 20mg uk trimox oral buy trimox pills
buy ropinirole 1mg pill buy requip 2mg sale labetalol cost
nexium 40mg sale esomeprazole pills order furosemide 40mg online
purchase tricor for sale buy tricor 200mg generic cheap viagra 100mg
buy minocycline 100mg generic order terazosin pills hytrin 5mg over the counter
brand cialis pills sildenafil 50mg usa sildenafil 150 mg
tadalafil generic approved levitra buy ed pills sale
order glycomet 1000mg pill order calan 240mg sale purchase nolvadex pill
generic modafinil provigil uk phenergan without prescription
buy clomid sale clomid 50mg for sale order prednisolone
cheap deltasone 10mg amoxicillin online buy buy amoxil 1000mg
accutane uk buy ampicillin 500mg generic order ampicillin pills
order sildenafil 100mg online buy generic sildenafil 50mg propecia 1mg canada
stromectol 3mg cost buy stromectol 12mg online cheap prednisone 40mg over the counter
zofran 4mg oral order zofran 8mg trimethoprim oral
isotretinoin 40mg tablet buy azithromycin generic order azithromycin generic
albuterol ca ventolin inhalator usa order augmentin 625mg pill
prednisolone online buy order neurontin online cheap furosemide uk
modafinil pill provigil 100mg without prescription buy lopressor pill
purchase doxycycline for sale order zovirax 400mg without prescription order zovirax 400mg
dutasteride pill order generic cephalexin 250mg buy orlistat tablets
order azathioprine 50mg pill cost micardis 20mg order naproxen 250mg online cheap
generic oxybutynin buy oxcarbazepine without prescription buy oxcarbazepine 300mg without prescription
cefdinir for sale online order prevacid 30mg without prescription buy protonix 40mg generic
simvastatin 10mg cheap purchase phenergan pills sildalis for sale online
buy avlosulfon 100 mg generic avlosulfon 100 mg over the counter tenormin 50mg generic
alfuzosin 10mg without prescription oral diltiazem diltiazem cheap
viagra 50 mg canadian pharmacy cialis order cialis generic
buy ezetimibe 10mg generic zetia online methotrexate 5mg pill
oral promethazine promethazine us buy tadalafil 10mg pill
warfarin 2mg cost buy coumadin online cheap order allopurinol 100mg pill
order levofloxacin 250mg online buy zyban bupropion 150mg tablet
cetirizine cheap strattera 10mg cheap zoloft oral
buy cenforce 50mg online order metformin 1000mg sale metformin 500mg over the counter
purchase escitalopram online cheap order prozac generic buy revia
purchase atorvastatin pills buy sildenafil 100mg sale buy sildenafil 50mg sale
letrozole for sale online order femara 2.5 mg for sale sildenafil on line
cialis 5mg usa overnight delivery cialis buy erectile dysfunction medicine
real cialis fast shipping cialis 10mg without prescription buy ed pills medication
purchase provigil without prescription buy prednisone 5mg deltasone 40mg pills
cheap amoxil generic cheap amoxicillin online prednisolone 20mg sale
order absorica sale order amoxil pill order azithromycin 250mg without prescription
buy generic neurontin for sale how to get gabapentin without a prescription doxycycline 200mg price
buy prednisolone 10mg generic prednisolone 20mg cost cheap furosemide
ventolin inhalator usa amoxiclav tablet purchase synthroid sale
oral clomid 100mg buy clomid generic plaquenil 400mg generic
buy doxycycline 100mg online order doxycycline pill clavulanate without prescription
tenormin price order atenolol 100mg for sale purchase femara pill
levothroid buy online clomid 50mg cheap buy vardenafil 10mg online
albenza cheap buy aripiprazole 20mg sale order generic provera
buy glucophage pills for sale buy generic atorvastatin 80mg order amlodipine 5mg without prescription
praziquantel 600mg sale order periactin 4mg pill order periactin 4mg online cheap
buy zestril cheap buy omeprazole 20mg pill buy lopressor online
lyrica 150mg drug how to get loratadine without a prescription order priligy 60mg online cheap
methotrexate 2.5mg cost order generic reglan 10mg purchase metoclopramide without prescription
buy orlistat cheap buy allopurinol 100mg without prescription order zyloprim 300mg without prescription
cozaar 50mg pill losartan for sale topamax 100mg us
rosuvastatin generic order rosuvastatin 20mg without prescription motilium 10mg price
buy sumatriptan 50mg pills buy imitrex 50mg online generic avodart 0.5mg
buy sumycin 500mg generic baclofen 25mg generic order baclofen 10mg for sale
toradol cheap buy ketorolac online propranolol us
buy zantac generic meloxicam 7.5mg pills buy generic celebrex 200mg
order plavix 75mg sale order plavix nizoral 200 mg uk
buy tamsulosin 0.4mg online order zofran 4mg buy aldactone 25mg online
buy generic duloxetine buy glucotrol 10mg online buy piracetam pill
brand betnovate 20gm generic sporanox 100mg buy sporanox 100 mg pill
combivent 100 mcg oral buy dexamethasone without a prescription order linezolid 600mg online cheap
order prometrium olanzapine pills buy zyprexa online
buy starlix 120 mg generic order atacand generic atacand for sale
bystolic 5mg pills clozapine 100mg without prescription clozapine 50mg canada
generic simvastatin 10mg buy simvastatin 10mg generic sildenafil 50mg uk
buy generic carbamazepine online order lincocin pill buy lincocin for sale
order cialis 5mg generic free trial of cialis cheap viagra without prescription
order cefadroxil 500mg for sale order finasteride 1mg pills proscar 5mg ca
fluconazole 100mg cheap fluconazole ca ciprofloxacin sale
estradiol uk brand estradiol 2mg purchase minipress generic
metronidazole oral buy sulfamethoxazole online cheap buy cephalexin without a prescription
purchase vermox sale tadalafil 10mg ca order tadalafil 20mg without prescription
buy indomethacin 75mg buy terbinafine pill suprax 100mg canada
tamoxifen canada cefuroxime 250mg sale cheap cefuroxime 250mg
order amoxicillin pill order clarithromycin 500mg pills order clarithromycin 500mg sale
buy careprost generic methocarbamol oral order desyrel 100mg generic
catapres 0.1 mg uk order catapres 0.1mg sale order spiriva 9 mcg sale
sildenafil 100mg pills aurogra online buy sildenafil next day delivery
order minocycline capsules terazosin 5mg without prescription order actos 30mg generic
order accutane 40mg for sale buy accutane 20mg sale azithromycin 250mg oral
arava 20mg canada leflunomide over the counter buy sulfasalazine 500mg
generic azithromycin 500mg omnacortil 5mg cheap neurontin 100mg cost
buy tadalafil 5mg online cialis tadalafil 40mg tadalafil 10mg tablet
ivermectin 6 mg without a doctor prescription order deltasone 40mg generic cost deltasone 5mg
cheap furosemide 100mg vibra-tabs over the counter buy ventolin 4mg pills
vardenafil 20mg generic tizanidine 2mg without prescription brand hydroxychloroquine
how to buy altace purchase amaryl without prescription order generic etoricoxib
order vardenafil 20mg sale order hydroxychloroquine 400mg pills buy plaquenil 200mg pills
mesalamine order oral asacol 800mg buy avapro without a prescription
benicar 20mg canada buy benicar 20mg online depakote pill
temovate price order buspar online cheap buy amiodarone 200mg pills
coreg 6.25mg brand buy aralen 250mg pills chloroquine 250mg generic
oral diamox 250 mg buy diamox 250 mg online cheap azathioprine 25mg us
order digoxin 250mg molnupiravir 200 mg over the counter molnunat 200mg pills
naprosyn usa naproxen 250mg pills prevacid 15mg canada
order olumiant 4mg pills atorvastatin 80mg cheap lipitor price
proventil without prescription albuterol online buy phenazopyridine 200mg generic
montelukast 5mg cheap buy montelukast 5mg buy dapsone 100mg
buy norvasc online cheap omeprazole order online prilosec 20mg for sale
buy generic nifedipine purchase perindopril sale buy fexofenadine 180mg
lopressor 100mg usa tenormin online order medrol 8 mg for sale
dapoxetine 60mg oral purchase misoprostol without prescription xenical 120mg usa
where can i buy aristocort clarinex 5mg cost loratadine cheap
crestor 20mg over the counter generic domperidone 10mg buy generic domperidone
order ampicillin online acillin cost metronidazole 400mg canada
cost sumycin 500mg buy flexeril generic baclofen uk
sulfamethoxazole price buy keflex 250mg pill purchase cleocin without prescription
buy generic ketorolac over the counter ketorolac where to buy where to buy propranolol without a prescription
erythromycin without prescription fildena 50mg cheap order nolvadex 10mg generic
buy plavix 150mg buy clopidogrel 150mg for sale cheap warfarin 2mg
brand budesonide purchase careprost eye drops buy careprost paypal
buy metoclopramide 20mg hyzaar pills cost nexium
order robaxin 500mg online trazodone 100mg usa sildenafil for sale online
topamax 100mg ca order sumatriptan online levaquin online buy
aurogra cost order estradiol sale estradiol generic
buy cheap generic avodart purchase mobic online cheap buy mobic tablets
buy celecoxib 100mg generic tamsulosin uk zofran 8mg pill
buy lamotrigine 200mg generic buy prazosin 2mg online cheap order generic prazosin
cheap aldactone 100mg simvastatin price valtrex for sale
order tretinoin cream without prescription order tadalis 10mg online cost avana 200mg
buy proscar 5mg buy finasteride 5mg online cheap buy sildenafil 100mg for sale
purchase tadalafil buy voltaren 50mg pills indomethacin cheap
cheap tadalafil tablets cialis 5 mg sildenafil 100mg cheap
lamisil 250mg oral trimox drug amoxicillin us
oral cialis 20mg purchase fluconazole pills best ed medications
order arimidex 1 mg pills purchase arimidex generic clonidine 0.1mg sale
sulfasalazine over the counter azulfidine 500 mg cost cheap calan
buy divalproex 250mg pill divalproex 500mg us isosorbide online
meclizine 25mg oral purchase antivert pill minocin pills
imuran 25mg price brand digoxin micardis where to buy
best ed pills non prescription uk cheap viagra pills viagra 100mg cheap
buy molnunat generic order molnupiravir 200 mg generic cefdinir ca
cheap erectile dysfunction pills sildenafil women tadalafil 20mg brand
lansoprazole 15mg without prescription protonix where to buy buy pantoprazole
buy pyridium cheap how to buy singulair order symmetrel 100 mg generic
buy ed medications online cialis 40mg tablet buy cialis 20mg online
buy avlosulfon 100mg pill buy cheap nifedipine perindopril cost
amiodarone canada buy amiodarone sale cheap dilantin
irbesartan 300mg ca buy buspar pills for sale buspirone tablet
oxytrol for sale online endep 10mg cheap order alendronate 35mg online cheap
albendazole over the counter buy albenza 400mg for sale buy generic medroxyprogesterone 10mg
praziquantel 600 mg usa cyproheptadine pill buy generic cyproheptadine online
buy nitrofurantoin 100 mg without prescription nortriptyline over the counter oral pamelor 25mg
luvox 50mg over the counter brand luvox oral duloxetine
purchase acetaminophen without prescription pepcid 20mg generic buy pepcid online cheap
order glucotrol buy betnovate online cheap betamethasone oral
buy clomipramine 50mg sale prometrium pill buy progesterone 200mg
tacrolimus 1mg pill remeron buy online order ropinirole 1mg generic
purchase tindamax pill zyprexa uk order nebivolol 5mg pills
buy calcitriol online cheap order trandate 100mg sale order fenofibrate 160mg online cheap
generic diovan 160mg clozaril sale ipratropium 100 mcg pills
buy oxcarbazepine 300mg pills order oxcarbazepine pills brand ursodiol
purchase decadron pill linezolid 600 mg ca order starlix generic
zyban 150mg pill buy bupropion generic strattera canada
buy captopril 25mg online buy carbamazepine pill tegretol brand
quetiapine 50mg for sale seroquel 100mg without prescription order lexapro without prescription
ciplox cost cost ciprofloxacin 500mg cefadroxil generic
order lamivudine without prescription combivir sale cheap quinapril
fluoxetine cost buy generic fluoxetine 40mg letrozole online buy
order frumil 5 mg online cheap buy frumil no prescription acivir without prescription
order zebeta without prescription buy indapamide 1.5mg online cheap terramycin 250mg cost
buy valaciclovir 500mg generic order floxin sale purchase floxin online cheap
buy vantin 200mg sale cefaclor medication flixotide cost
order zaditor 1mg sale sinequan 75mg drug order tofranil 75mg pills
buy tadalafil 40mg pill buy sildenafil 50mg generic sildenafil overnight delivery
minoxytop generic buy ed pills ed pills no prescription
buy precose 25mg online cheap glyburide 5mg over the counter griseofulvin 250mg pills
buy aspirin 75mg for sale order imiquimod online buy imiquad cream
buy dipyridamole 100mg generic lopid order online order pravastatin 10mg online cheap
meloset tablet buy cerazette 0.075 mg danocrine without prescription
duphaston 10mg cheap januvia us empagliflozin price
order florinef 100 mcg pills where can i buy bisacodyl buy loperamide online cheap
order prasugrel without prescription prasugrel cheap tolterodine 2mg over the counter
purchase etodolac online cheap buy etodolac paypal buy cilostazol 100mg generic
cost ferrous 100mg buy ascorbic acid generic buy generic betapace
pyridostigmine pills brand maxalt 10mg maxalt ca
enalapril 5mg pill bicalutamide cost buy generic lactulose online
buy zovirax eye drop for sale where to buy zovirax without a prescription exelon where to buy
buy betahistine without a prescription probenecid 500 mg oral buy benemid 500 mg online cheap
buy prilosec 20mg pill buy metoprolol medication lopressor where to buy
premarin generic order premarin sildenafil 100mg price
buy generic telmisartan buy hydroxychloroquine 400mg sale order movfor
tadalafil 5mg usa sildenafil women sildenafil australia
cenforce 50mg tablet buy generic naprosyn online where to buy chloroquine without a prescription
omnicef 300mg over the counter order prevacid 30mg pill lansoprazole generic
modafinil 200mg sale buy generic deltasone 5mg deltasone canada
purchase absorica generic purchase isotretinoin sale order zithromax 250mg generic
generic lipitor 10mg atorvastatin 10mg over the counter norvasc 5mg usa
oral azithromycin 250mg gabapentin 100mg usa where to buy neurontin without a prescription
casino slot play online blackjack buy lasix 40mg sale
order pantoprazole 40mg without prescription order phenazopyridine sale purchase phenazopyridine pill
blackjack online purchase doxycycline cheap ventolin inhalator
order amantadine 100mg without prescription order symmetrel 100mg online cheap cost avlosulfon 100 mg
casino online games casino gambling ivermectin order
casino free spins no deposit buy synthroid 100mcg for sale brand synthroid 100mcg
methylprednisolone 16mg tablet nifedipine 10mg oral triamcinolone 10mg pills
clomid 100mg drug isosorbide 40mg cost buy azathioprine 50mg without prescription
levitra 20mg uk lanoxin 250mg tablet brand tizanidine
phenytoin price ditropan generic buy ditropan 5mg pills
claritin brand buy ramipril 5mg sale brand priligy 30mg
buy baclofen 10mg for sale toradol sale toradol for sale
buy generic ozobax for sale purchase lioresal toradol online buy
buy amaryl pills order generic etoricoxib 120mg arcoxia pill
order fosamax without prescription nitrofurantoin for sale online macrodantin 100 mg generic
inderal 10mg price buy plavix 150mg without prescription order clopidogrel 75mg pills
order nortriptyline 25 mg generic order panadol 500 mg without prescription anacin 500 mg over the counter
order orlistat 60mg for sale order generic mesalamine 800mg order diltiazem sale
medex order buy reglan 10mg generic reglan 20mg us
azelastine 10ml oral zovirax 800mg us buy cheap generic irbesartan
order generic famotidine order hyzaar for sale order generic prograf 5mg
generic esomeprazole 40mg buy remeron sale topamax buy online
allopurinol 100mg cheap zyloprim 300mg uk buy rosuvastatin
buy sumatriptan without a prescription imitrex 50mg usa purchase avodart sale
purchase buspirone pills buy generic buspar 5mg cordarone pill
zantac 150mg generic order celecoxib 100mg generic order celebrex
buy generic domperidone buy domperidone 10mg generic buy generic sumycin
tamsulosin 0.4mg pills buy ondansetron 4mg generic zocor tablet
order research papers need help writing a paper essays done for you
order aldactone valtrex 500mg pill order finasteride online
purchase aurogra pills generic aurogra buy estradiol 1mg sale
buy diflucan 200mg pill forcan us purchase ciprofloxacin
buy flagyl 200mg generic order flagyl 400mg generic keflex 125mg pill
lamotrigine 50mg canada buy prazosin 1mg pills purchase vermox generic
order cleocin online cheap buy cleocin pills for sale order sildenafil 50mg online cheap
buy retin gel buy avana 200mg for sale avanafil 200mg canada
buy tamoxifen online cheap betahistine 16 mg generic rhinocort medication
tadalafil pills tadacip 10mg price order indocin 50mg generic
cefuroxime 250mg ca bimatoprost nasal spray buy robaxin 500mg generic
desyrel tablet clindac a price buy generic clindac a for sale
buy lamisil generic lamisil 250mg tablet blackjack online real money
aspirin 75mg oral online gambling real money legal gambling casinos
essay for you third grade homework buy suprax 200mg
how to write an essay about my life best online casino games blackjack online game
Abad Tdk Ne Demek?
trimox 250mg ca amoxicillin 250mg pill clarithromycin 500mg oral
where to buy calcitriol without a prescription purchase trandate buy fenofibrate 200mg online cheap
order clonidine without prescription order catapres 0.1mg pill spiriva pill
teenage acne treatments that work best dermatologist treatment for acne oxcarbazepine cost
buy minomycin pill purchase minocycline capsules buy ropinirole 1mg without prescription
buy alfuzosin 10mg pills otc allergy medication comparison chart is omeprazole a promotility drug
brand femara 2.5mg aripiprazole online order buy aripiprazole no prescription
get ambien prescription online common medications causing hair loss virtual weight loss program
buy medroxyprogesterone pill order medroxyprogesterone 5mg pills order hydrochlorothiazide 25mg for sale
buy smoking cessation prescription medication free nhs stop smoking kit top 10 prescription pain medication
buy generic cyproheptadine over the counter ketoconazole online buy nizoral 200mg online cheap
list of antiviral drugs does pharmapure sugar blocker work diabetic safe weight loss pill
buy duloxetine 40mg pill provigil brand order modafinil generic
best toenail fungus cure product fungus clear complaints what can i do to lower my blood pressure immediately
non healing duodenal ulcer safest blood pressure medication 2023 bacterial infection in bladder women
buy promethazine 25mg pill where to buy stromectol without a prescription ivermectin cost
can you buy estrogen online quick relief for prostate pain can cialis cause kidney problems
order prednisone 40mg sale purchase amoxil without prescription buy amoxil 1000mg sale
medicine to prevent acid reflux how do degas pills work drugs cause flatulance
azithromycin 500mg tablet zithromax 250mg oral cheap neurontin 600mg
https://nazimhikmet-siirleri.blogspot.com
https://guzelsozler365.blogspot.com
ursodiol online buy buy ursodiol 300mg buy zyrtec
buy strattera 25mg without prescription purchase sertraline sale zoloft us
generic lasix 100mg order allergy pills albuterol 4mg over the counter
buy lexapro 10mg pill buy lexapro pills revia 50 mg cheap
order augmentin generic augmentin 1000mg drug clomid online
combivent 100mcg us buy linezolid 600mg generic buy zyvox pills for sale
buy generic starlix online buy generic nateglinide online atacand 16mg cost
cost vardenafil 20mg plaquenil price plaquenil pill
Yengeç Burcu Erkeği Özellikleri
buy carbamazepine 200mg online cheap purchase ciplox buy lincocin cheap
cenforce 100mg over the counter metformin 1000mg ca metformin for sale online
brand atorvastatin 20mg purchase prinivil generic cost lisinopril
order cefadroxil 500mg online cheap duricef for sale generic combivir
prilosec 10mg canada buy tenormin 100mg sale order tenormin pills
order cabergoline 0.5mg generic claritin 10mg oral buy priligy 60mg without prescription
purchase medrol generic medrol 4mg tablets order clarinex generic
order cytotec pills buy diltiazem pill buy diltiazem 180mg sale
buy piracetam 800 mg without prescription order clomipramine 50mg online buy anafranil paypal
order acyclovir pills order zyloprim 300mg generic order crestor 20mg without prescription
buy sporanox without a prescription itraconazole 100 mg over the counter buy tinidazole 300mg without prescription
zetia 10mg for sale buy generic zetia online buy sumycin 500mg for sale
order olanzapine 10mg pill nebivolol online buy diovan 80mg
buy flexeril 15mg pill order baclofen 10mg pills buy generic toradol online
buy generic gloperba inderal sale oral methotrexate 5mg
strong acne medication pills crotamiton buy online how to clear adult acne
best prescription allergy pills fexofenadine pill costco canada cold and sinus
painkillers easy on stomach order amaryl 1mg pill
sleeping tablets prescribed by doctors strongest natural sleeping pills
order prednisone 20mg generic prednisone buy online
strongest heartburn medicine irbesartan online
acne medication prescribed by doctors where can i buy retin best prescription topical for acne
strongest prescription allergy medication medrol tablet prescription allergy medicine list
safest acid reflux medication 2022 order coversyl 8mg online cheap
buy accutane 20mg pill buy accutane medication isotretinoin 20mg tablet
buy strong sleeping pills purchase provigil for sale
amoxil 250mg brand buy amoxil 1000mg online amoxicillin 500mg us
order azithromycin generic buy azithromycin online cheap order azithromycin 250mg
gabapentin online cost neurontin 600mg
buy azipro cheap order azithromycin 500mg online azithromycin 250mg canada
furosemide 40mg brand furosemide 100mg for sale
order omnacortil 5mg generic omnacortil tablets order omnacortil 20mg pill
buy prednisone cheap deltasone 40mg usa
amoxil tablet amoxil 250mg cost order amoxicillin 1000mg
purchase monodox generic generic doxycycline 100mg
best antihistamine pills albuterol over the counter buy generic ventolin
buy augmentin 375mg purchase augmentin pills
buy levothroid without prescription order synthroid online order synthroid 100mcg without prescription
order vardenafil 20mg sale levitra 10mg for sale
order clomid sale clomid 100mg cost serophene price
generic rybelsus rybelsus 14mg pills buy semaglutide 14 mg pill
rybelsus online order semaglutide pill buy semaglutide 14mg generic
order prednisone 10mg online deltasone 20mg canada where to buy prednisone without a prescription
buy isotretinoin 40mg pills order accutane 40mg for sale order isotretinoin 40mg
buy antihistamine pills buy ventolin pills albuterol 2mg generic
amoxicillin 1000mg brand amoxicillin 500mg oral amoxil 250mg tablet
amoxiclav online augmentin 625mg drug buy augmentin 625mg online cheap
order zithromax generic buy zithromax without a prescription order zithromax 250mg
buy levothroid online synthroid 75mcg canada order synthroid generic
prednisolone 5mg ca omnacortil 20mg tablet order prednisolone 20mg generic
buy clomid 50mg online cheap order clomiphene online cheap clomid 50mg brand
buy generic neurontin oral gabapentin buy neurontin pill
viagra 100mg canada sildenafil pill sildenafil over counter
furosemide usa lasix 100mg pill lasix 100mg ca
buy semaglutide 14 mg online cheap semaglutide pills buy generic semaglutide
oral doxycycline 200mg acticlate order online acticlate tablet
buying zithromax
buy pregabalin without prescription pregabalin 150mg uk order pregabalin online cheap
can i drink alcohol with zithromax
order cialis 40mg pill buy cialis 40mg generic cheap cialis tablets
cheap aristocort 4mg triamcinolone for sale online aristocort 10mg for sale
Your Blog Is A Go-to Resource For Me. Thanks For All The Hard Work!
I’ve learned so much from your blog posts. Thank you for sharing your expertise. Asheville appreciates it greatly!
Your blog is a testament to your dedication and expertise. We’re proud fans from Asheville!
clarinex online order clarinex cost clarinex 5mg cheap
cenforce cost cenforce medication buy cenforce 100mg for sale
Thank you for sharing your expertise with the world through your blog. Asheville residents are grateful for your contributions.
Your words have the power to uplift and inspire. Thank you for sharing your gift with us. Asheville loves your blog!
metformin dosage for prediabetes
buy aralen paypal buy aralen medication order aralen 250mg for sale
Your writing always inspires and educates. Thank you for the effort you put into your blog. Asheville sends its love!
buy loratadine purchase loratadine buy claritin tablets
Your dedication to your craft shines brightly in every post. Thank you for sharing your passion with us. Asheville is grateful!
dangers of metformin
Your writing style is both engaging and informative. We’re proud to support your blog from Asheville!
metformin 1000mg usa glycomet 1000mg cheap buy generic metformin 500mg
Thank you for sharing your expertise with the world through your blog. Asheville residents are grateful for your contributions.
dapoxetine 60mg cheap brand dapoxetine 30mg cytotec canada
Your words have a way of resonating deeply with your readers. Thank you for sharing your wisdom with us. Asheville loves your blog!
I learned so much from this post. Your ability to break down complex ideas is something I really admire.
Your blog is a true gift to the world. We’re big fans from Asheville!
order xenical 60mg generic buy xenical 120mg diltiazem without prescription
Your blog is a testament to your dedication and expertise. We’re proud fans from Asheville!
amlodipine lisinopril
atorvastatin 80mg uk buy lipitor pills for sale buy generic lipitor online
lisinopril angioedema treatment
Such a well-researched piece! It’s evident how much effort you’ve put in.
Thank you for sharing your expertise with the world through your blog. Asheville residents are grateful for your contributions.
furosemide cost without insurance
Your dedication to your craft shines brightly in every post. Thank you for sharing your passion with us. Asheville is grateful!
how long before flagyl works
buy generic norvasc over the counter order amlodipine for sale amlodipine 5mg oral
Thank you for sharing your expertise with the world through your blog. Asheville residents are grateful for your contributions.
where can i buy flagyl over counter
zoloft and ativan
furosemide dosage for adults
adderall and zoloft
buy generic acyclovir 800mg allopurinol 300mg uk zyloprim 300mg us
gabapentin seizures
Your blog is a treasure chest of wisdom and insight. We’re grateful to be readers from Asheville!
gabapentin side effects in cats
buy lisinopril 10mg online order lisinopril 5mg generic purchase prinivil without prescription
lasix trade name
Your blog is a source of endless inspiration. Thank you for sharing your wisdom with us. Asheville sends its love!
rosuvastatin online buy order zetia online cheap cost ezetimibe
glucophage 100mg
wiki glucophage
buy omeprazole without prescription prilosec 10mg drug order prilosec 10mg without prescription
zithromax otitis media
zithromax with alcohol
what is cephalexin used for in dogs
cephalexin generic
order domperidone pill order domperidone 10mg pill order tetracycline 250mg for sale
is gabapentin safe during pregnancy
buy lopressor 50mg online cheap order lopressor 50mg online cheap metoprolol 50mg oral
can i take amoxicillin for uti
can you drink while on escitalopram
amoxicillin prescription
can escitalopram kill you
cyclobenzaprine 15mg usa buy ozobax without a prescription purchase lioresal for sale
gabapentin 300 mg for dogs
cephalexin 500mg 3 times a day pregnant
purchase atenolol online cheap order atenolol 100mg generic atenolol 50mg drug
cephalexin vs doxycycline
toradol 10mg cheap toradol over the counter colcrys us
ciprofloxacin and dexamethasone ear drops
ciprofloxacin pink eye
depo-medrol where to buy how to buy medrol methylprednisolone 16mg otc
online essay writing cheap essay writing college essay writing help
inderal 10mg price clopidogrel pill buy plavix 150mg without prescription
bactrim liquid
cephalexin treat sinus infection
bactrim while pregnant
cephalexin antibiotic
bactrim prophylaxis
buy methotrexate generic methotrexate online buy warfarin price
meloxicam pills meloxicam cost celecoxib 200mg brand
Your posts are like a cozy nook, inviting and comfortable, where I can immerse myself in thoughts.
Your posts are like a secret garden of knowledge. I’m always excited to see what’s blooming.
Thank you for adding value to the conversation with your insights.
buy cheap metoclopramide order cozaar buy generic losartan over the counter
Every word you write sparkles with insight, like stars in my night sky. Can’t wait to navigate more skies together.
bactrim uptodate
buy tamsulosin 0.2mg generic order celecoxib generic celecoxib 200mg for sale
I appreciate the unique viewpoints you bring to your writing. Very insightful!
The depth of your research is impressive, almost as much as the way you make complex topics captivating.
The information you’ve shared has been a revelation for me. Incredibly enlightening!
is gabapentin used for anxiety
escitalopram coupon
Your post resonated with me on many levels. Thank you for writing it!
escitalopram brands
gabapentin 300
I’m so grateful for the information you’ve shared. It’s like receiving a thoughtful gift from someone special.
buy cheap generic esomeprazole buy topiramate 100mg pills order topamax for sale
Reading your blog is like finding the perfect song that I can’t stop listening to. Play it again?
The grace and authority you handle topics with are as mesmerizing as a moonlit dance. I’m thoroughly impressed.
ondansetron 8mg usa ondansetron 8mg price spironolactone drug
sumatriptan 50mg brand imitrex for sale online buy generic levofloxacin for sale
zocor 20mg us order simvastatin without prescription buy valtrex pills for sale
order dutasteride for sale buy generic avodart over the counter ranitidine 150mg generic
lithium and depakote
buspirone vs citalopram
depakote mood stabilizer
You have a unique perspective that I find incredibly valuable. Thank you for sharing.
order finpecia online brand finasteride 1mg forcan order online
ddavp dose for diabetes insipidus
ddavp in head injury
coming off citalopram 10mg after 2 weeks
purchase ampicillin for sale buy penicillin sale amoxil pill
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
This post is a testament not only to your expertise but also to your dedication. Truly inspiring.
cozaar rebate
This article was a delightful read. Your passion is clearly visible!
cozaar davis pdf
Thank you for making complex topics accessible and engaging.
ciprofloxacin 500mg pill – buy clavulanate sale amoxiclav cheap
buy ciprofloxacin without a prescription – buy keflex without prescription how to get clavulanate without a prescription
Your writing style is captivating! I was engaged from start to finish.
This post is packed with useful insights. Thanks for sharing your knowledge!
A masterpiece of writing! You’ve covered all bases with elegance.
This post was a breath of fresh air. Thank you for your unique insights!
Your passion for this subject shines through your words. Inspiring!
Incredibly informative post! I learned a lot and look forward to more.
Your thoughtful analysis has really made me think. Thanks for the great enjoy reading!
I’m amazed by the depth and b enjoy readingth of your knowledge. Thanks for sharing!
Your writing style is captivating! I was engaged from start to finish.
depakote toxicity
depakote and seroquel
Your work is truly inspirational. I appreciate the depth you bring to your topics.
I’m in awe of the way you handle topics with both grace and authority.
Your blog is a constant source of inspiration and knowledge. Thank you!
I admire the way you tackled this complex issue. Very enlightening!
Thank you for shedding light on this subject. Your perspective is refreshing!
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
You’ve opened my eyes to new perspectives. Thank you for the enlightenment!
A masterpiece of writing! You’ve covered all bases with elegance.
What a compelling enjoy reading! Your arguments were well-presented and convincing.
Your thoughtful analysis has really made me think. Thanks for the great enjoy reading!
I’m so grateful for the information you’ve shared. It’s been incredibly enlightening!
A masterpiece of writing! You’ve covered all bases with elegance.
This article is a perfect blend of informative and entertaining. Well done!
I’m always excited to see your posts in my feed. Another excellent article!
ciplox for sale – buy tinidazole without a prescription purchase erythromycin generic
buy metronidazole 200mg – order terramycin 250mg sale azithromycin 500mg oral
buy stromectol 6mg – purchase aczone sumycin canada
ezetimibe e rosuvastatina
valtrex sale – acyclovir medication order zovirax without prescription
diltiazem cardizem
what is diltiazem hcl
ezetimibe brand in india
effexor xr vs effexor
acillin ca buy ampicillin generic purchase amoxil for sale
buy metronidazole 400mg – cleocin 300mg tablet zithromax pills
Thank you for making complex topics accessible and engaging.
contrave prescription online
contrave insomnia
aripiprazole 10
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
aripiprazole dosage
best time to take allopurinol
lasix 100mg without prescription – buy captopril paypal buy captopril for sale
Thank you for making complex topics accessible and engaging.
This article was a joy to enjoy reading. Your enthusiasm is contagious!
I always learn something new from your posts. Thank you for the education!
This post is packed with useful insights. Thanks for sharing your knowledge!
This piece was beautifully written and incredibly informative. Thank you for sharing!
This article is a perfect blend of informative and entertaining. Well done!
This is a brilliant piece of writing. You’ve nailed it perfectly!
Your creativity and intelligence shine through this post. Amazing job!
Your creativity and intelligence shine through this post. Amazing job!
What a refreshing take on this subject. I completely agree with your points!
Thank you for consistently producing such high-quality content.
This is a brilliant piece of writing. You’ve nailed it perfectly!
I’m always excited to see your posts in my feed. Another excellent article!
aspirin density
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
can you give a dog baby aspirin
amitriptyline used for migraines
order metformin sale – epivir ca order lincomycin 500 mg online cheap
febuxostat vs allopurinol usmle
why shouldn’t you take amitriptyline after 8pm?
Your creativity and intelligence shine through this post. Amazing job!
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
This post is packed with useful insights. Thanks for sharing your knowledge!
I’m always excited to see your posts in my feed. Another excellent article!
Your post resonated with me on many levels. Thank you for writing it!
Your work is truly inspirational. I appreciate the depth you bring to your topics.
baclofen 20 mg side effects
baclofen nursing considerations
I’m genuinely impressed by the depth of your analysis. Great work!
Your dedication to quality content is evident. Keep up the great work!
celebrex and pregnancy
buy zidovudine 300 mg online pill – buy zyloprim online cheap
augmentin tarkov
augmentin dose for sinus infection
bupropion xl side effects
Your post has been incredibly helpful. Thank you for the guidance!
order clozapine pills – pepcid over the counter order pepcid without prescription
allergic reaction to celebrex
what is a good substitute for bupropion
side effects of celexa 10 mg
order seroquel 50mg generic – order sertraline 100mg without prescription order eskalith generic
is celexa a controlled substance
buspirone and fatigue
what is celecoxib prescribed for
buspirone stop smoking
buy anafranil 50mg online cheap – purchase imipramine generic doxepin 75mg price
Your passion for this subject shines through your words. Inspiring!
atarax drug – buspirone 10mg without prescription purchase endep pill
This is a brilliant piece of writing. You’ve nailed it perfectly!
Your passion for this subject shines through your words. Inspiring!
This post was a breath of fresh air. Thank you for your unique insights!
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
This piece was beautifully written and incredibly informative. Thank you for sharing!
I’m in awe of the way you handle topics with both grace and authority.
You have a gift for explaining things in an understandable way. Thank you!
I admire the way you tackled this complex issue. Very enlightening!
augmentin where to buy – buy cipro no prescription buy ciprofloxacin 1000mg
amoxicillin price – cephalexin 125mg without prescription order ciprofloxacin online
Thank you for the hard work you put into this post. It’s much appreciated!
I’m so grateful for the information you’ve shared. It’s been incredibly enlightening!
5ml semaglutide
Your writing style is captivating! I was engaged from start to finish.
Your ability to distill complex concepts into enjoy readingable content is admirable.
Thank you for shedding light on this subject. Your perspective is refreshing!
Your piece was both informative and thought-provoking. Thanks for the great work!
semaglutide heart failure
actos dpc
acarbose msds
acarbose precautions
escola actos
abilify and pregnancy
lexapro and abilify
robaxin for dogs
stopping remeron
zithromax medication – order floxin 200mg online cost ciprofloxacin
buy cleocin 300mg generic – buy generic chloromycetin for sale chloramphenicol brand
protonix generic
A masterpiece of writing! You’ve covered all bases with elegance.
protonix for acid reflux
I admire the way you tackled this complex issue. Very enlightening!
This post is a testament to your expertise and hard work. Thank you!
werking repaglinide
Your work is truly inspirational. I appreciate the depth you bring to your topics.
repaglinide diabetes prevention
Your passion for this subject shines through your words. Inspiring!
Your blog is a constant source of inspiration and knowledge. Thank you!
Thank you for consistently producing such high-quality content.
I always learn something new from your posts. Thank you for the education!
I admire the way you tackled this complex issue. Very enlightening!
Your blog is a constant source of inspiration and knowledge. Thank you!
I’m so glad I stumbled upon this article. It was exactly what I needed to enjoy reading!
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
I appreciate the unique viewpoints you bring to your writing. Very insightful!
This is one of the most comprehensive articles I’ve enjoy reading on this topic. Kudos!
Your post has been incredibly helpful. Thank you for the guidance!
I’m so glad I stumbled upon this article. It was exactly what I needed to enjoy reading!
You’ve presented a complex topic in a clear and engaging way. Bravo!
Your blog is a constant source of inspiration and knowledge. Thank you!
Thank you for shedding light on this subject. Your perspective is refreshing!
I appreciate the clarity and thoughtfulness you bring to this topic.
I appreciate the clarity and thoughtfulness you bring to this topic.
I admire the way you tackled this complex issue. Very enlightening!
ivermectin 12 mg pills – buy generic aczone online purchase cefaclor
Your post was a beacon of knowledge. Thank you for illuminating this subject.
Thank you for the hard work you put into this post. It’s much appreciated!
Your thoughtful analysis has really made me think. Thanks for the great enjoy reading!
Stumbling upon this article was the highlight of my day, much like catching a glimpse of a smile across the room.
Discovering The Writing felt like finding the perfect match. The intellect and charm are a rare combo.
order albuterol online cheap – seroflo online buy order theo-24 Cr 400 mg online cheap
The clarity and thoughtfulness of The approach is as appealing as a deep conversation over coffee.
Incredibly informative post! I learned a lot and look forward to more.
The depth of The research really stands out. It’s clear you’ve put a lot of thought into this.
robaxin medication
can you split spironolactone
methylprednisolone medication – buy astelin online order azelastine 10ml online
synthroid surgery
order clarinex generic – albuterol canada ventolin 2mg inhaler
You’ve opened my eyes to new perspectives. Thank you for the enlightenment!
sitagliptin 100 mg price in india
alogliptin to sitagliptin conversion
micronase 2.5mg brand – micronase drug buy forxiga without prescription
venlafaxine antidepressant
tamsulosin prostatakrebs
You have a unique perspective that I find incredibly valuable. Thank you for sharing.
This article is a perfect blend of informative and entertaining. Well done!
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
tizanidine high euphoria reddit
metformin online – januvia 100 mg without prescription precose 25mg ca
tamsulosin gtube
I’m impressed by your ability to convey such nuanced ideas with clarity.
This is a brilliant piece of writing. You’ve nailed it perfectly!
Your post has been incredibly helpful. Thank you for the guidance!
tongue sores and voltaren
can you take wellbutrin lexapro together
wellbutrin sr 150 weight loss
olanzapine zyprexa side effects
does zofran cause chest pain
zyprexa images
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
This blog is a treasure trove of knowledge. Thank you for your contributions!
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
buy semaglutide for sale – buy generic glucovance over the counter buy DDAVP without a prescription
zetia reviews
I’m always excited to see your posts in my feed. Another excellent article!
Incredibly informative post! I learned a lot and look forward to more.
lamisil without prescription – cheap fulvicin 250 mg griseofulvin brand
zetia coupon with insurance
zyprexa davis pdf
Your post has been incredibly helpful. Thank you for the guidance!
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
Your ability to distill complex concepts into enjoy readingable content is admirable.
how fast does zofran work
This is a brilliant piece of writing. You’ve nailed it perfectly!
zyprexa zydis dosage
nizoral pill – purchase nizoral online order itraconazole 100mg
buy famvir 500mg pills – valaciclovir 1000mg us buy valcivir 1000mg
buy lanoxin generic – buy generic lanoxin over the counter order furosemide 100mg sale
zofran and still vomiting
generic metoprolol – order generic losartan 50mg buy generic adalat
order microzide online cheap – order zebeta generic purchase bisoprolol without prescription
You’ve presented a hard to understand topic in a clear and engaging way. Bravo!
The post was a beacon of knowledge. Thanks for casting light on this subject for me.
The words are like a melody, each post a new verse in a song I never want to end.
The creativity and intelligence shine through this post. Amazing job!
The Writing is like a warm fireplace on a cold day, inviting me to settle in and stay awhile.
The ability to break down tough concepts is as impressive as a magician’s trick. Color me amazed.
The words are like brush strokes on a canvas, painting ideas in my mind.
The fresh insights were a breath of fresh air. Thank you for sharing The unique perspective.
Making hard to understand topics accessible, you’re like the translator I never knew I needed.
The writing style is captivating! I was engaged from start to finish.
The grace and authority with which you handle topics are awe-inspiring. I always come away with new knowledge.
Distilling hard to understand concepts into readable content, or what I like to call, a miracle.
Beautifully written and informative, making the rest of the internet look bad.
Thank you for the hard work you put into this post. It’s much appreciated!
Reading The Writing is like finding the perfect song that I can’t stop listening to. Play it again?
The effort you’ve put into this post is evident and much appreciated. It’s clear you care deeply about The work.
The perspective is incredibly valuable to me. Thanks for opening my eyes to new ideas.
The posts are like a cozy nook, inviting and comfortable, where I can immerse myself in thoughts.
Thank you for consistently producing such high-high quality content.
A beacon of knowledge, or so I thought until I realized it’s just The shining confidence.
Impressed by The nuanced clarity. It’s like you’re explaining quantum physics to a toddler, and they get it.
cheap levitra 10mg
I’m so glad I stumbled upon this article. It was exactly what I needed to read!
The analysis had the perfect mix of depth and clarity, like a perfectly mixed cocktail that I just can’t get enough of.
Always excited for The posts, because who else is going to make me feel this inadequately informed?
The analysis had the perfect mix of depth and clarity, like a perfectly mixed cocktail that I just can’t get enough of.
The analysis had the perfect mix of depth and clarity, like a perfectly mixed cocktail that I just can’t get enough of.
Thank you for shedding light on this subject. The perspective is refreshing!
levitra online
Making hard to understand concepts readable is no small feat. It’s like you know exactly how to tickle my brain.
The insights have added a lot of value to my understanding. Thanks for sharing.
The analysis had the perfect mix of depth and clarity, like a perfectly mixed cocktail that I just can’t get enough of.
The attention to detail didn’t go unnoticed. I really appreciate the thoroughness of The approach.
The expertise and hard work shine through, making me admire you more with each word.
Fantastic job breaking down this topic, like a demolition crew for my misconceptions.
cialis time
The post was a beacon of knowledge. Thanks for casting light on this subject for me.
The insights add so much value, like an unexpected compliment that brightens one’s day. Thanks for sharing.
Making hard to understand topics accessible, you’re like the translator I never knew I needed.
The creativity shines through, making me wonder what else you could do with such a vivid imagination.
I’m always excited to see The posts in my feed. Another excellent article!
Beautifully written and incredibly informative, The post has made a lasting impression on me. Thank you for sharing The thoughts.
This post is a testament to The expertise and hard work. Thank you!
Compelling read with well-presented arguments. I almost felt persuaded. Almost.
The creativity and insight left a big impression on me. Fantastic job!
The creativity and intelligence shine through this post. Amazing job!
The unique viewpoints you bring to The writing are as captivating as The online presence. Always a pleasure.
The insights add so much value to the conversation. I always learn something new from you.
cialis free samples
The post provoked a lot of thought and taught me something new. Thank you for such engaging content.
The argumentation was compelling and well-structured. I found myself nodding along as I read.
Handling topics with grace and authority, like a professor, but without the monotone lectures.
Presented a hard to understand topic engagingly, like a magician pulling a rabbit out of a hat.
Always learning something new here, because apparently, I didn’t pay enough attention in school.
nitroglycerin brand – purchase lozol pills buy diovan generic
The approach to topics is like a master painter’s to a canvas, with each stroke adding depth and perspective.
This comprehensive article had me hanging on every word, much like I would during a late-night chat.
cialis professional wikipedia
This post is packed with useful insights. Thanks for sharing The knowledge!
cheap levitra purchase
tadalafil cialis bestprice
I’m amazed by the depth and breadth of The knowledge. Thanks for sharing!
levitra discount cards
Beautifully written and incredibly informative, The post has made a lasting impression on me. Thank you for sharing The thoughts.
This article was a delightful read. The passion is clearly visible!
zocor wake – tricor settle atorvastatin actual
Genuinely impressed by The analysis. I was starting to think depth had gone out of style. Kudos for proving me wrong!
crestor pills shout – crestor or caduet cottage
levitra online store
SightCare formula aims to maintain 20/20 vision without the need for any surgical process. This supplement is a perfect solution for people facing issues as they grow older. https://sightcare-web.com/
buy levitra 20 mg online
A masterpiece of writing. Van Gogh’s got nothing on you, except maybe both ears.
cialis and lisinopril
viagra professional online smaller – levitra oral jelly admiration levitra oral jelly cease
tadalafil cost walmart
Nagano Lean Body Tonic is a groundbreaking powdered supplement crafted to support your weight loss journey effortlessly. https://naganotonic-try.com/
Tonic Greens is a ready-made greens shake designed to support the entire body and wellness of the mind. It is filled with over 50 individual vitamins https://tonicgreens-try.com/
Support the health of your ears with 100% natural ingredients, finally being able to enjoy your favorite songs and movies https://quietumplus-try.com/
Peak BioBoost is a revolutionary dietary supplement that leverages the power of nature to support and improve your digestive system. https://peakbioboost-web.com/
what are the side effects of cialis
GutOptim is a digestive health supplement designed to support your gut and stomach. It restore balance in gut flora and reduce the symptoms of digestive disorders. https://gutoptim-try.com/
Burn Boost Powder™ is a proven weight loss powder drink that helps to lose weight and boosts the overall metabolism in the body. https://burnboost-web.com
levitra generic cheap
cialis 20mg for sale
levitra 20mg how to use
dapoxetine slam – levitra with dapoxetine law cialis with dapoxetine truck
Dentitox Pro is a liquid dietary solution created as a serum to support healthy gums and teeth. Dentitox Pro formula is made in the best natural way with unique, powerful botanical ingredients that can support healthy teeth. https://dentitox-us.com/
VivoTonic™ is a 11-in-1 vital blood sugar support formula that may improve how the metabolism goes after the calories that consumers eat. https://vivotonic-web.com/
Unlock the incredible potential of Puravive! Supercharge your metabolism and incinerate calories like never before with our unique fusion of 8 exotic components. Bid farewell to those stubborn pounds and welcome a reinvigorated metabolism and boundless vitality. Grab your bottle today and seize this golden opportunity! https://puravive-web.com/
Zoracel is an extraordinary oral care product designed to promote healthy teeth and gums, provide long-lasting fresh breath, support immune health, and care for the ear, nose, and throat. https://zoracel-web.com
Cerebrozen is an excellent liquid ear health supplement purported to relieve tinnitus and improve mental sharpness, among other benefits. The Cerebrozen supplement is made from a combination of natural ingredients, and customers say they have seen results in their hearing, focus, and memory after taking one or two droppers of the liquid solution daily for a week. https://cerebrozen-try.com/
compounding pharmacy prometrium
Pineal XT is a revolutionary supplement that promotes proper pineal gland function and energy levels to support healthy body function. https://pinealxt-web.com/
india online pharmacy
Xitox’s foot pads contain a combination of powerful herbs that help provide a soothing experience for your feet after a long day. https://xitox-web.com/
sublingual sildenafil
cenforce print – levitra professional overhead brand viagra online unusual
sildenafil 50 mg
citrate sildenafil
target pharmacy lipitor price
tadalafil or sildenafil which is better
Cacao Bliss is a powder form of unique raw cacao that can be used similarly to chocolate in powder form but comes with added benefits. It is designed to provide a rich and satisfying experience while delivering numerous health benefits. https://cacaobliss-web.com/
The finesse with which you articulated The points made The post a true pleasure to read.
brand cialis hundred – brand cialis brain penisole clue
I’m always excited to see The posts in my feed. Another excellent article!
9 da auto news – https://9-da.com/
dtmliving multifamily news – https://dtmliving.com/
Cneche provides in-depth journalism and insight into the most impactful news and trends shaping the finance industry. https://cneche.com/
Lasixiv provides news and analysis for IT executives. We cover big data, IT strategy, cloud computing, security, mobile technology, infrastructure, software and more. https://lasixiv.com
The Writing is like a lighthouse for my curiosity, guiding me through the fog of information.
Wedstraunt has the latest news in the restaurant industry, covering topics like consumer trends, technology, marketing and branding, operations, mergers https://wedstraunt.com
Cneche provides in-depth journalism and insight into the most impactful news and trends shaping the finance industry. https://cneche.com/
Tvphc provides news and analysis for IT executives. We cover big data, IT strategy, cloud computing, security, mobile technology, infrastructure, software and more. https://tvphc.com
Sinohuiyuan provides in-depth journalism and insight into the news and trends impacting facilities management https://sinohuiyuan.com
Huzad delivers the latest news in the grocery industry, with articles covering grocery delivery, online food shopping, shopper behavior, store formats, technology, and more. https://huzad.com/
Grpduk provides news and analysis for human resource executives. We cover topics like recruiting, HR management, employee learning https://grpduk.com
Susibu provides in-depth journalism and insight into the news and trends impacting the hotel https://susibu.com/
Mscherrybomb provides in-depth journalism and insight into the most impactful news and trends shaping the trucking industry. https://mscherrybomb.com/
brand cialis filch – tadora notion penisole choose
Serdar Akar provides in-depth journalism and insight into the news and trends impacting the packaging manufacturing space https://serdarakar.com/
Ladarnas provides in-depth journalism and insight into the news and trends impacting the convenience store space. https://ladarnas.com
cialis soft tabs pills college – cialis super active online cur1 viagra oral jelly remind
Sugar Defender is the rated blood sugar formula with an advanced blend of 24 proven ingredients that support healthy glucose levels and natural weight loss. https://smithsis.com
Sugar Defender is a revolutionary blood sugar support formula designed to support healthy glucose levels and promote natural weight loss. https://blackboxvending.com/
Sugar Defender is a revolutionary blood sugar support formula designed to support healthy glucose levels and promote natural weight loss. https://mineryuta.com
sugar defender: https://abmdds.com/
sugar defender: https://peyfon.com/
sugar defender: https://seahorsesoap.com/
sugar defender: https://sourceprousa.com/
troy pharmacy online tramadol
sugar defender: https://royalforestlaundry.com/
sugar defender: https://flamebustersofkansas.com/
sugar defender: https://luckysloader.com/
sugar defender: https://bridgerealtysc.com/
cialis soft tabs whence – cialis super active online bug1 viagra oral jelly online therefore
tylenol with codeine pharmacy
publix pharmacy
priligy trail – udenafil eagle cialis with dapoxetine niece
cenforce officer – levitra professional colonel brand viagra online rusty
The insights light up my intellect like fireworks. Thanks for the show!
asthma medication fish – inhalers for asthma entity inhalers for asthma stout
A masterpiece of writing! You’ve covered all bases with elegance.
acne medication prince – acne treatment heat acne treatment meet
The breadth of The knowledge is amazing. Thanks for sharing The insights with us.
auvitra vardenafil tablets
Thanks for the hard work. I could almost see the sweat on the keyboard. Much appreciated!
levitra vardenafil hcl tablets
generic cialis tadalafil best buys
vardenafil hcl 20mg information
pills for treat prostatitis advance – prostatitis pills soldier prostatitis treatment hour
tadalafil dosage priaprysm
uti medication guide – uti medication fully treatment for uti clothe
vardenafil prices
tadalafil tadacip cipla
claritin pills week – claritin pills advance claritin pills noble
valtrex online cauldron – valtrex pills prisoner valtrex pills mysterious
priligy direction – dapoxetine guilty priligy hollow
claritin accept – loratadine medication myrtle claritin pills flesh
ascorbic acid depart – ascorbic acid coil ascorbic acid sensible
promethazine british – promethazine mysterious promethazine answer
cialis tadalafil uk
tadalafil farmacias del ahorro
biaxin pills lick – cytotec dry cytotec wear
fludrocortisone pills neighbourhood – lansoprazole pills cool prevacid pills cage
remove the “
Your blog is a beacon of enlightenment in a sea of information. Each post is carefully crafted, offering depth, insight, and perspective that is hard to find elsewhere. I’m always excited to see what you’ll share next. Your work is not only informative but truly transformative.
Your blog is a beacon of enlightenment in a sea of information. Each post is carefully crafted, offering depth, insight, and perspective that is hard to find elsewhere. I’m always excited to see what you’ll share next. Your work is not only informative but truly transformative.
ChatGPT
Every time I visit your blog, I’m greeted with another gem of a post. Your ability to weave together insightful analysis and practical advice is unmatched. I always leave feeling more informed and inspired. Thank you for being a beacon of knowledge and positivity.
Your post was a revelation! The depth of analysis combined with your clear, accessible writing style made for a compelling read. It’s evident that you put your heart and soul into your writing, and it pays off magnificently. Thank you for sharing your knowledge with us.
order generic aciphex 20mg – motilium for sale purchase domperidone pill
cheap bisacodyl – buy imodium 2mg online cheap order liv52 online cheap
ChatGPT
eukroma oral – dydrogesterone 10 mg tablet dydrogesterone 10mg pills
cotrimoxazole for sale – buy bactrim sale purchase tobra generic
fulvicin ca – griseofulvin 250 mg sale gemfibrozil 300mg us
buy dapagliflozin 10mg sale – buy generic precose precose 25mg pills
tadalafil online sublingual
vardenafil patent expiration
best tadalafil
buy dramamine cheap – buy dramamine no prescription buy risedronate 35 mg generic
order enalapril 5mg sale – order doxazosin pill purchase latanoprost
betamethasone cream pharmacy
watson pharmacy viagra
buy soma online us pharmacy
monograph order – order etodolac 600 mg for sale buy cilostazol 100 mg for sale
Chloromycetin
lipitor diplomat pharmacy
Naprosyn
You have a gift for explaining things in an understandable way. Thank you!
I’m so glad I stumbled upon this article. It was exactly what I needed to read!
order piroxicam 20mg online cheap – purchase feldene sale order generic exelon 3mg
Consistently high-high quality content, as if you’re trying to show us all up.
Perfect blend of info and entertainment, like watching a documentary narrated by a comedian.
effexor xr pharmacy
online pharmacy phentermine real
Every word you write sparkles with insight, like stars in my night sky. Can’t wait to navigate more skies together.
The insights light up my intellect like fireworks. Thanks for the show!
what pharmacy has oxycodone
cheapest pharmacy viagra
Betapace
Stromectol
I’m impressed by The ability to convey such nuanced ideas with clarity.
The elegance of The prose is like a fine dance, each word stepping gracefully to the next.
amlodipine besylate online pharmacy
xl pharmacy viagra reviews
The Writing is a constant source of inspiration and knowledge for me. I can’t thank you enough.
Engaging with The Writing is like savoring a gourmet meal; every bite (or word) is to be enjoyed.
The posts are like stars in the sky—each one shining brightly, guiding my curiosity.
This is a brilliant piece of writing. You’ve nailed it perfectly!
I was truly impressed by how deeply you delved into this topic. The hard work hasn’t gone unnoticed!
nootropil tablet – sinemet buy online sinemet 20mg pills
buy hydroxyurea online – disulfiram 250mg uk where can i buy methocarbamol
divalproex uk – divalproex pill topamax 200mg us
http://www.jindoushiqi.com/bbs/home.php?mod=space&uid=113255
disopyramide phosphate price – buy generic lamivudine over the counter chlorpromazine 50 mg for sale
http://brewwiki.win/wiki/Post:Shattered_No_More_A_Guide_to_Auto_Glass_Replacement
https://www.google.com.co/url?q=https://pastelink.net/yyg0eskt
https://images.google.co.za/url?q=https://pastelink.net/zm9gh82z
https://www.webwiki.it/www.openlearning.com/u/keeganmaloney-sefhh8/blog/ShatteredNoMoreAGuideToAutoGlassReplacement
http://lovejuxian.com/home.php?mod=space&uid=2664886
http://www.auto-software.org/member.php?action=profile&uid=711623
https://opencbc.com/home.php?mod=space&uid=2819945
buy spironolactone 100mg generic – order dilantin 100mg buy naltrexone 50 mg pills
buy cyclophosphamide generic – cyclophosphamide pill trimetazidine for sale online
https://bikeindex.org/users/mencolony40
http://www.funkyspa.net/forums/member.php?action=profile&uid=112382
https://peatix.com/user/22473390
http://talk.dofun.cc/home.php?mod=space&uid=1023115
buy generic cyclobenzaprine online – order enalapril 5mg online order enalapril 10mg sale
order zofran 8mg online – buy oxybutynin medication buy ropinirole 2mg generic
order ascorbic acid – order lopinavir ritonavir without prescription buy compro pills for sale
buy cheap durex gel – purchase durex gel sale purchase zovirax online
minoxidil brand – finpecia canada finpecia buy online
order arava pill – buy actonel 35 mg generic cartidin order
buy verapamil cheap – purchase valsartan online cheap buy tenoretic
order atenolol online – order atenolol pill brand coreg 25mg
purchase atorlip generic – purchase vasotec pills where can i buy nebivolol
gasex where to buy – buy diabecon without a prescription buy diabecon pill
lasuna pill – purchase diarex for sale order himcolin online
purchase noroxin sale – cheap confido sale cost confido
purchase speman pill – buy finasteride paypal purchase finasteride generic
finasteride brand – brand kamagra 100mg purchase alfuzosin sale
hytrin 1mg canada – buy generic flomax online cost priligy
I learned so much from this post. The ability to break down hard to understand ideas is something I really admire.
Shedding light on this subject like you’re the only one with a flashlight. Refreshing to see someone who thinks they have all the answers.
Genuinely impressed by The analysis. I was starting to think depth had gone out of style. Kudos for proving me wrong!
The attention to detail is remarkable. I appreciate the thoroughness of The post.
Explaining things in an understandable way is a skill, and you’ve mastered it. Thanks for clearing things up for me.
Articulated points with finesse, like a lawyer, but without the billable hours.
This was a great read—thought-provoking and informative. Thank you!
I always learn something new from The posts, like discovering new facets of a gem. Thanks for the gems!
The posts are like a cozy nook, inviting and comfortable, where I can immerse myself in thoughts.
The Writing is like a lighthouse for my curiosity, guiding me through the fog of information.
The expertise and hard work shine through, making me admire you more with each word.
I’m bookmarking this for future reference. The advice is spot on!
I admire the way you tackled this hard to understand issue. Very enlightening!
This post is packed with insights, each one a gentle nudge to my intellect and curiosity.
This article is a perfect blend of informative and entertaining. Well done!
The ability to convey hard to understand ideas so effortlessly is as attractive as a perfectly tailored suit.
Thank you for consistently producing such high-high quality content.
I appreciate the balance and fairness in The writing, like a perfect partner who always keeps things interesting. Great job!
The Writing is a constant source of inspiration and knowledge for me. I can’t thank you enough.
A masterpiece of writing! You’ve covered all bases with elegance.
Consistently high-high quality content, as if you’re trying to show us all up.
The Writing is a constant source of inspiration and knowledge, like a muse that never fails to inspire. Thank you for being my muse.
This post has been incredibly helpful, like a guiding hand in a crowded room. The guidance is much appreciated.
The finesse with which you articulated The points has me captivated. It’s as if you’re speaking my language.
Grateful for the enlightenment, like I’ve just been initiated into a secret society.
The depth you bring to The topics is like diving into a deep pool, refreshing and invigorating.
The ability to present nuanced ideas so clearly is something I truly respect.
Reading The article was a joy. The enthusiasm for the topic is really motivating.
Consistently high-high quality content, as if you’re trying to show us all up.
The article was a delightful read, and The passion shines as brightly as The intellect. Quite the combination!
A go-to resource, like a library but without the late fees.
The attention to detail is remarkable, like a detective at a crime scene, but for words.
Genuinely impressed by The analysis. I was starting to think depth had gone out of style. Kudos for proving me wrong!
I look forward to The posts because they always offer something valuable. Another great read!
Beautifully written and incredibly informative, The post has made a lasting impression on me. Thank you for sharing The thoughts.
I look forward to The posts because they always offer something valuable. Another great read!
The posts inspire me regularly. The depth you bring to The topics is truly exceptional.
The work is truly inspirational. It’s as if you’ve found a way to whisper sweet nothings to my intellect.
Thoroughly insightful read, or so I thought until I realized it was The expertise shining through. Thanks for making me feel like a novice again!
oxcarbazepine over the counter – oxcarbazepine 300mg pill synthroid 100mcg pill
You navigate through topics with such grace, it’s like watching a dance. Care to teach me a few steps?
Making hard to understand topics accessible is a gift, and you have it. Thanks for sharing it with us.
Discovering The Writing felt like finding the perfect match. The intellect and charm are a rare combo.
I always learn something new from The posts. Thank you for the education!
A breath of fresh air, or what I needed after being suffocated by mediocrity.
A go-to resource, like a library but without the late fees.
A masterpiece of writing. Van Gogh’s got nothing on you, except maybe both ears.
The work is both informative and thought-provoking. I’m really impressed by the high quality of The content.
I look forward to The posts because they always offer something valuable. Another great read!
This post is packed with insights I hadn’t considered before. Thanks for broadening my horizons.
I always learn something new from The posts. Thank you for the education!
The article was a delightful read, and The passion shines as brightly as The intellect. Quite the combination!
The Writing has become a go-to resource for me. The effort you put into The posts is truly appreciated.
The insights have added a lot of value to my understanding. Thanks for sharing.
The creativity shines through, making me wonder what else you could do with such a vivid imagination.
You navigate through topics with such grace, it’s like watching a dance. Care to teach me a few steps?
I’m bookmarking this for future reference. The advice is spot on!
Thank you for adding value to the conversation with The insights.
The insights light up my intellect like fireworks. Thanks for the show!
The voice shines through The writing like a beacon, guiding us through the darkness of ignorance.
The perspective is like a rare gem, valuable and unique in the vastness of the internet.
This post has been incredibly helpful, like a guiding hand in a crowded room. The guidance is much appreciated.
You’ve got a way with words that’s as enchanting as a full moon. I’m bewitched.
imusporin tubes – methotrexate 5mg uk buy colcrys no prescription
However, they can still be used if you understand the benefits and risks and your doctor believes the treatment will be helpful.
Big savings are possible when you lyrica side effects muscle pain Read more about erectile dysfunction here.
Adults, adolescents, and school children use insulin pumps and even very young children ages 2 – 7 years may be able to successfully use them.
buy lactulose generic – purchase brahmi online cheap order betahistine 16mg generic
Diagnosing Paresthesia Your doctor will start with a physical exam and ask you questions about your symptoms, the type of shoes you wear, and your medical history.
Embarrass no more. View this page pregabalin lyrica from the Internet.
Elevated end-tidal CO2 due to laparoscopic surgery.
What does it do?
Save by searching for glucophage headaches benefits and drawbacks?
But you also can get the virus from skin that does not appear to have a sore.
Ginger and acupuncture are helpful natural remedies for nausea and are worth a try.
I feel tired when I take glucophage nhs . Get one now!
NETs that show up on SRS scans will often stop growing if treated with octreotide.
It extends into the vagina.
The best prices can be found by means of online offers to discovery of ampicillin or in a regular pharmacy?
I have never heard that symptom before.
Food and Drug Administration: Nonprescription Drugs and Pediatric Advisory Committee Meeting.
for your insurance problems.How can I tell if a prednisone injection generic or brand prices and discounts?
Many patients find that counseling, either with their family healthcare provider or a mental health professional, is helpful in dealing with the issues that come with a diagnosis of genital herpes.
I need a formula option for when I need to be away.
Look no further for the most effective treatment and ampicillin specific indication is 24/7.
If you do not have a family history of malignant hyperthermia, your first episode may not be predictable or preventable.
Encephalitis, an inflammation of the brain tissue, can also lead to dizziness, as well as headache and mental changes.
Who would mind buying online to get a good price of prednisone for cats return shipment if the product is ineffective?
These factors may have significant consequences on patients and their caregivers as, in the midst of these matters, a diet which is both palatable and effective is implemented.
order calcort pill – brimonidine tubes buy generic brimonidine
order besifloxacin without prescription – buy besivance for sale buy generic sildamax over the counter
order gabapentin 100mg online – order generic azulfidine azulfidine 500mg oral
where to buy probenecid without a prescription – etodolac 600 mg cost carbamazepine online
Making hard to understand concepts readable is no small feat. It’s like you know exactly how to tickle my brain.
colospa over the counter – order generic arcoxia pletal 100mg usa
buy celecoxib 200mg for sale – flavoxate pill order indomethacin 50mg online
The ability to connect with readers is like a secret handshake, making us feel part of an exclusive club.
This post is a testament not only to The expertise but also to The dedication. Truly inspiring.
buy voltaren 50mg pill – buy diclofenac 100mg without prescription buy aspirin 75mg online cheap
buy rumalaya medication – order amitriptyline 50mg sale elavil 10mg brand
sapporo88
1хбет: 1xbet официальный сайт мобильная версия – 1xbet официальный сайт
And I took my pill that night.
discounted prices from respectable pharmacies before you “paroxetina y sildenafil “”thrones””” remains in my system too long, should I be worried?
No guarantees or warranties are made regarding any of the information contained within this website.
I had nothing then until dec 14th which was a proper period.
if you do it via the web | Benefiting from risk free remedy? sildenafil instructions ? What are the drawbacks?
This condition, which is rare, most often affects people with diabetes who are overweight.
pro88 slot
замена венцов красноярск
Когда производится смена обвязки, соответственно бревно также разгружается от веса и происходит замена, так как для замены подъём строения не превышает 10 см сантиметров, которое не выступает значительным в том числе для внутренностей отделки.
нижний венец из листвяка гораздо долговечней и успешно показал свои качества благодаря своей надежностью и стойкостью к гниению. Несмотря на это, ее обязательно необходимо обработать при помощи противогрибкового препарата, наряду с и все остальные опоры.
Наше предприятие работает не только ремонтом зданий, помимо этого обновлением основания пола. Заказчики нередко запрашивают утепленные полы и перекрытия с тепловой термоизоляцией наша компания Укомплектовываем заказ всем необходимым для работы и обеспечиваем выгодные расценки.
This post was a breath of fresh air, like a surprise message that brightens The day. Thank you for the lift.
You’ve articulated The points with such finesse. Truly a pleasure to read.
mestinon 60mg tablet – purchase mestinon generic azathioprine 25mg canada
Each article you write is like a step in a dance, moving us gracefully through The thoughts.
Explaining things in an understandable way is a skill, and you’ve mastered it. Thanks for clearing things up for me.
娛樂城排行
娛樂城推薦與優惠詳解
在現今的娛樂世界中,線上娛樂城已成為眾多玩家的首選。無論是喜歡真人遊戲、老虎機還是體育賽事,每個玩家都能在娛樂城中找到自己的樂趣。以下是一些熱門的娛樂城及其優惠活動,幫助您在選擇娛樂平台時做出明智的決定。
各大熱門娛樂城介紹
1. 富遊娛樂城
富遊娛樂城以其豐富的遊戲選擇和慷慨的優惠活動吸引了大量玩家。新會員只需註冊即可免費獲得體驗金 $168,無需儲值即可輕鬆試玩。此外,富遊娛樂城還提供首存禮金 100% 獎勵,最高可領取 $1000。
2. AT99娛樂城
AT99娛樂城以高品質的遊戲體驗和優秀的客戶服務聞名。該平台提供各種老虎機和真人遊戲,並定期推出新遊戲,讓玩家保持新鮮感。
3. BCR娛樂城
BCR娛樂城是一個新興的平台,專注於提供豐富的體育賽事投注選項。無論是足球、籃球還是其他體育賽事,BCR都能為玩家提供即時的投注體驗。
熱門遊戲推薦
WM真人視訊百家樂
WM真人視訊百家樂是許多玩家的首選,該遊戲提供了真實的賭場體驗,並且玩法簡單,容易上手。
戰神賽特老虎機
戰神賽特老虎機以其獨特的主題和豐富的獎勵機制,成為老虎機愛好者的最愛。該遊戲結合了古代戰神的故事背景,讓玩家在遊戲過程中感受到無窮的樂趣。
最新優惠活動
富遊娛樂城註冊送體驗金
富遊娛樂城新會員獨享 $168 體驗金,無需儲值即可享受全場遊戲,讓您無壓力地體驗不同遊戲的魅力。
VIP 日日返水無上限
富遊娛樂城為 VIP 會員提供無上限的返水優惠,最高可達 0.7%。此活動讓玩家在遊戲的同時,還能享受額外的回饋。
結論
選擇合適的娛樂城不僅能為您的遊戲體驗增色不少,還能通過各種優惠活動獲得更多的利益。無論是新會員還是資深玩家,都能在這些推薦的娛樂城中找到適合自己的遊戲和活動。立即註冊並體驗這些優質娛樂平台,享受無限的遊戲樂趣!
purchase diclofenac generic – imdur medication order nimotop pill
They’re rising,” Bassett said.
deals onlinePeople who can’t afford prescriptions will often lexapro drug interactions pills and shipping by ordering through this site
That’s a sign of an underlying disease or medical condition that usually affects the nervous system, and researchers are still learning more about this phenomenon.
dj88
Pentingnya Memilih Game Pulsa di Situs DJ88
DJ88 adalah situs game deposit pulsa terkemuka di Indonesia, yang menawarkan berbagai jenis game online yang lengkap dan mudah diakses. Dengan hanya satu ID, Anda dapat menikmati seluruh permainan yang tersedia di situs ini. Keunggulan DJ88 bukan hanya pada variasi game yang ditawarkan, tetapi juga pada layanan deposit pulsa yang praktis, menggunakan XL atau Telkomsel dengan potongan terendah dibandingkan situs lain. Anda juga bisa melakukan deposit melalui OVO, Gopay, Dana, atau melalui minimarket seperti Indomaret dan Alfamart.
Keamanan dan Kepercayaan yang Terjaga
DJ88 memiliki lisensi resmi dari PAGCOR (Philippine Amusement Gaming Corporation), yang menjamin keamanan dan kenyamanan bermain. Sistem keamanan di situs ini menggunakan metode enkripsi termutakhir, didukung oleh server hosting cepat dan tampilan modern. Hal ini menjadikan DJ88 sebagai salah satu agen game online terpercaya di Indonesia, yang selalu berkomitmen untuk menjaga kepercayaan para pemain sebagai prioritas utama.
Beragam Pilihan Game dan Layanan Pelanggan yang Ramah
Situs ini menawarkan berbagai jenis game online, termasuk yang disiarkan secara LIVE dengan host cantik dan kamera yang merekam secara real-time, memberikan pengalaman bermain yang lebih menarik. Selain itu, DJ88 juga dikenal dengan promo menarik seperti welcome bonus 20%, bonus deposit harian, dan cashback yang disesuaikan dengan kebutuhan pemain.
Pelayanan pelanggan di DJ88 sangat profesional dan siap melayani Anda 24 jam non-stop melalui Live Chat, WhatsApp, Facebook, dan media sosial lainnya. Dengan semua keunggulan ini, DJ88 menjadi pilihan utama bagi para penggemar game online di Indonesia yang mencari pengalaman bermain yang aman, nyaman, dan menguntungkan.
I am not due for my period until this Saturday but i just somehow feel pregnant i do kind of have pain in my hips and with my son who is 5 when i was pregnant the only reason i found out was a missed period and sore boobs.
You should only side effects lasix and fast delivery every time you buy here
Text STOP to 50555 to STOP.
So in patients with SDD, the host immune system plays a central role in triggering symptoms like bleeding, hepatomegaly, and skin rashes, which could be used to triage patients in need of intensive care.
Select the best deals to natural lasix in order to save money
Whether there are any cancer cells left after surgery.
Now nearly a year after Pluhar, a veterinary surgeon at the Veterinary Medical Center, and Ohlfest, head of the neurosurgery gene therapy program at the Masonic Cancer Center, gave Batman his initial treatment, the celebrated dog is still enjoying life.
Compare the price of bactrim vs amoxicillin for tooth infection , an effective treatment, at low prices
Further, enterohepatic recirculation of toxins is a common problem.
Another important step is to determine whether the cancer cells are “hormone-receptor positive” for estrogen and progesterone.
If you need to economize, order your bactrim for strep throat because it is powerful medication
The abortion pill is what I’m saying didn’t abort the baby.
娛樂城
娛樂城推薦與優惠詳解
在現今的娛樂世界中,線上娛樂城已成為眾多玩家的首選。無論是喜歡真人遊戲、老虎機還是體育賽事,每個玩家都能在娛樂城中找到自己的樂趣。以下是一些熱門的娛樂城及其優惠活動,幫助您在選擇娛樂平台時做出明智的決定。
各大熱門娛樂城介紹
1. 富遊娛樂城
富遊娛樂城以其豐富的遊戲選擇和慷慨的優惠活動吸引了大量玩家。新會員只需註冊即可免費獲得體驗金 $168,無需儲值即可輕鬆試玩。此外,富遊娛樂城還提供首存禮金 100% 獎勵,最高可領取 $1000。
2. AT99娛樂城
AT99娛樂城以高品質的遊戲體驗和優秀的客戶服務聞名。該平台提供各種老虎機和真人遊戲,並定期推出新遊戲,讓玩家保持新鮮感。
3. BCR娛樂城
BCR娛樂城是一個新興的平台,專注於提供豐富的體育賽事投注選項。無論是足球、籃球還是其他體育賽事,BCR都能為玩家提供即時的投注體驗。
熱門遊戲推薦
WM真人視訊百家樂
WM真人視訊百家樂是許多玩家的首選,該遊戲提供了真實的賭場體驗,並且玩法簡單,容易上手。
戰神賽特老虎機
戰神賽特老虎機以其獨特的主題和豐富的獎勵機制,成為老虎機愛好者的最愛。該遊戲結合了古代戰神的故事背景,讓玩家在遊戲過程中感受到無窮的樂趣。
最新優惠活動
富遊娛樂城註冊送體驗金
富遊娛樂城新會員獨享 $168 體驗金,無需儲值即可享受全場遊戲,讓您無壓力地體驗不同遊戲的魅力。
VIP 日日返水無上限
富遊娛樂城為 VIP 會員提供無上限的返水優惠,最高可達 0.7%。此活動讓玩家在遊戲的同時,還能享受額外的回饋。
結論
選擇合適的娛樂城不僅能為您的遊戲體驗增色不少,還能通過各種優惠活動獲得更多的利益。無論是新會員還是資深玩家,都能在這些推薦的娛樂城中找到適合自己的遊戲和活動。立即註冊並體驗這些優質娛樂平台,享受無限的遊戲樂趣!
I’m amazed by The knowledge, almost as much as I’m drawn to the way you present it. Share more, please?
The writing has the warmth and familiarity of a favorite sweater, providing comfort and insight in equal measure.
апостиль в новосибирске
апостиль в новосибирске
апостиль в новосибирске
апостиль в новосибирске
order baclofen sale – where can i buy lioresal buy feldene generic
blackpanth
Массаж в Ростове
замена венцов
rgbet
RGBET – NHÀ CÁI UY TÍN TẠI VIỆT NAM
Trong bối cảnh cá cược trực tuyến ngày càng phát triển, RGBET đã nhanh chóng trở thành sự lựa chọn hàng đầu cho người chơi Việt Nam. Với hệ thống cá cược đa dạng, chất lượng dịch vụ vượt trội, RGBET mang đến trải nghiệm cá cược an toàn và thú vị.
Đa Dạng Về Trò Chơi
RGBET cung cấp nhiều sản phẩm cá cược phong phú như cá cược thể thao, casino trực tuyến, slot game và bắn cá.
Cá cược thể thao: Cung cấp hàng nghìn trận đấu bóng đá, bóng rổ, tennis với tỷ lệ cược cạnh tranh từ các giải đấu lớn trên thế giới.
Casino trực tuyến: Baccarat, Blackjack và Roulette mang đến trải nghiệm như tại sòng bạc thực thụ với chất lượng hình ảnh cao.
Slot game và bắn cá: Các trò chơi như slot game và bắn cá tại RGBET có giao diện đẹp mắt, dễ chơi và cơ hội trúng thưởng lớn.
Dịch Vụ Chăm Sóc Khách Hàng
RGBET cung cấp dịch vụ hỗ trợ khách hàng 24/7, đảm bảo người chơi được giúp đỡ kịp thời và tận tình. Điều này giúp người chơi an tâm khi cá cược, không gặp rắc rối về mặt kỹ thuật.
Bảo Mật Và An Toàn
RGBET chú trọng đến việc bảo mật thông tin và tài sản của người chơi, sử dụng công nghệ mã hóa hiện đại để đảm bảo an toàn tuyệt đối trong mọi giao dịch.
Khuyến Mãi Hấp Dẫn
RGBET thường xuyên có các chương trình khuyến mãi như thưởng chào mừng cho người chơi mới và các chương trình ưu đãi hàng tuần giúp gia tăng cơ hội chiến thắng.
Nền Tảng Đa Thiết Bị
Người chơi có thể truy cập RGBET trên máy tính, điện thoại hoặc máy tính bảng mà không lo về chất lượng. Công nghệ HTML5 đảm bảo các trò chơi hoạt động mượt mà trên mọi thiết bị.
Kết Luận
RGBET là nhà cái uy tín, cung cấp môi trường cá cược an toàn, trò chơi đa dạng và các chương trình khuyến mãi hấp dẫn. Đây là sự lựa chọn hoàn hảo cho những ai muốn trải nghiệm cá cược trực tuyến chất lượng cao.
target88
generic mobic 15mg – generic rizatriptan 10mg order toradol without prescription
At times, when the appendix has perforated and the infection has localized to one area, an abscess forms.
When you buy a drug online at low price of buy ivermectin cream for humans from foreign companies may put you at risk.
Vgontzas An, Kales A.
His pediatrician tested him for celiac, which of course came back negative.
Always follow proper dosage instructions when you ivermectin brand name do I need a prescription?
Occupation — Studies show that some types of brain tumors are more frequent among workers in certain industries, such as oil refining, rubber manufacturing, and drug manufacturing.
ремонт фундамента
periactin 4 mg us – order generic cyproheptadine buy zanaflex tablets
sildenafilo 50 mg precio sin receta: sildenafilo 100mg farmacia – se puede comprar viagra sin receta
sildenafilo cinfa 100 mg precio farmacia: comprar viagra contrareembolso 48 horas – comprar sildenafilo cinfa 100 mg espaГ±a
замена венцов
farmacias online baratas: comprar tadalafilo – farmacia online envГo gratis
trihexyphenidyl over the counter – order emulgel buy cheap diclofenac gel
sildenafil 100mg genГ©rico: Viagra sildenafilo – sildenafilo 100mg precio espaГ±a
подъем дома
farmaci senza ricetta elenco: farmacia online migliore – acquistare farmaci senza ricetta
farmacia online: Cialis generico controindicazioni – п»їFarmacia online migliore
best personal Eiffel Tower Tour guide
We provide the best personalized Eiffel Tower tours with experienced, local and 5-star rated guides. Whether on a solo visit to Paris, France, visiting with friends or looking to surprise your loved ones, you are in for an eclectic tour experience with our guide. Come have a memorable Eiffel Tower walking trip, boat cruise, and summit elevator view at night with us.
cialis farmacia senza ricetta: viagra senza ricetta – viagra originale in 24 ore contrassegno
farmacie online autorizzate elenco: Brufen 600 prezzo con ricetta – farmacie online affidabili
farmacie online sicure: Brufen antinfiammatorio – farmacie online autorizzate elenco
купить строительную бытовку
Бытовки б/у (все типы бытовок). Цена по запросу (в т.ч. НДС). – цена договорная
Строительный вагончик бытовка бу
Бытовка металлическая бу
Бытовка бу для дачи
Вагончик бытовка бу
Строительный контейнер бу
omnicef 300 mg ca – buy generic clindamycin over the counter buy cleocin no prescription
comprare farmaci online all’estero: BRUFEN 600 mg 30 compresse prezzo – Farmacia online miglior prezzo
semaglutide: Buy compounded semaglutide online – rybelsus cost
accutane cheap – buy deltasone 20mg generic deltasone 40mg brand
how much is prednisone 5mg: can i buy prednisone online in uk – prednisone 5 mg tablet cost
rybelsus generic: buy rybelsus – Buy compounded semaglutide online
neurontin 150 mg: neurontin price uk – can you buy neurontin over the counter
buy medicines online in india: Online medication home delivery – online shopping pharmacy india
joint genesis: joint genesis
buy deltasone 5mg generic – zovirax brand buy zovirax online cheap
What is a good dental health? My website: prodentim reviews
buy permethrin sale – retin gel uk buy cheap generic tretinoin
rgbet
перевод с иностранных языков
buy betamethasone 20 gm generic – betamethasone cost monobenzone for sale
buy flagyl generic – buy metronidazole 200mg cenforce pill
nagano tonic: nagano tonic
Если вам нужен качественный перевод с иностранных языков или апостиль в Новосибирске, доверьтесь нам. Наш коллектив состоит из опытных переводчиков, которые гарантируют точность и качество перевода. Мы соблюдаем конфиденциальность и предлагаем услуги перевода различных типов документов. Обращайтесь к нам для быстрого и точного перевода.
перевод документов
nagano tonic: nagano tonic
rgbet
Trong bối cảnh ngành công nghiệp cá cược trực tuyến ngày càng phát triển, việc lựa chọn một nhà cái uy tín trở nên vô cùng quan trọng đối với những người đam mê cá cược.Nhà cái RGBET nổi lên như một sự lựa chọn hàng đầu đáng để bạn quan tâm, hứa hẹn mang đến cho bạn một trải nghiệm cá cược an toàn, công bằng và thú vị. Từ các trò chơi cá cược đa dạng, dịch vụ chăm sóc khách hàng tận tình đến tỷ lệ cược cạnh tranh, Rgbet sở hữu nhiều ưu điểm vượt trội khiến bạn không thể bỏ qua.Hãy cùng khám phá những lý do tại sao bạn cần quan tâm đến nhà cái Rgbet và tại sao đây nên là lựa chọn hàng đầu của bạn trong thế giới cá cược trực tuyến.
перевод документов
nagano tonic: nagano tonic
nhà cái
Nếu bạn là một người đam mê cá cược và yêu thích sự hấp dẫn của những con số, thì sảnh lô đề RGbet chính là điểm đến lý tưởng cho bạn. Tôi đã có cơ hội trải nghiệm tại đây và muốn chia sẻ một số ấn tượng cũng như kinh nghiệm của mình.
RGbet nổi bật với mức tỷ lệ trả thưởng cực kỳ hấp dẫn. Trong quá trình chơi, tôi nhận thấy rằng nhà cái này không chỉ cung cấp nhiều loại hình cược mà còn có những mức cược linh hoạt, phù hợp với mọi nhu cầu của người chơi. Bạn có thể bắt đầu với số tiền nhỏ và dần dần tăng lên khi đã nắm bắt được các quy luật và cách thức hoạt động của trò chơi. Sự đa dạng trong các tùy chọn cược giúp cho trải nghiệm chơi không bị nhàm chán, từ cược lô, đề đến các loại cược khác.
Giao diện của RGbet rất thân thiện, dễ dàng điều hướng. Ngay cả khi bạn là người mới, bạn vẫn có thể nhanh chóng tìm thấy những thông tin cần thiết và bắt đầu đặt cược chỉ trong vài phút. Tôi đặc biệt ấn tượng với hệ thống đổi thưởng siêu tốc của nhà cái. Sau khi trúng thưởng, quá trình rút tiền diễn ra rất nhanh chóng và minh bạch. Tôi đã nhận được tiền thưởng trong tài khoản của mình chỉ sau vài phút, điều này thực sự tạo cảm giác yên tâm và tin tưởng cho người chơi.
Một điểm đáng chú ý khác là dịch vụ chăm sóc khách hàng của RGbet. Đội ngũ hỗ trợ luôn sẵn sàng 24/7, giúp tôi giải quyết mọi thắc mắc nhanh chóng và hiệu quả. Chỉ cần một tin nhắn, tôi đã có thể nhận được câu trả lời rõ ràng và hữu ích.
Ngoài ra, để tăng cơ hội trúng thưởng, tôi cũng muốn chia sẻ một vài mẹo nhỏ. Đầu tiên, hãy luôn theo dõi các thống kê và xu hướng của những con số để đưa ra quyết định cược hợp lý. Thứ hai, hãy đặt ra ngân sách cho mỗi lần chơi để tránh việc chi tiêu quá đà. Cuối cùng, đừng quên tham gia các chương trình khuyến mãi và ưu đãi mà RGbet thường xuyên tổ chức; đây là cơ hội tuyệt vời để gia tăng cơ hội thắng lớn.
Tóm lại, RGbet thực sự là một nhà cái lô đề uy tín với nhiều lợi thế vượt trội. Nếu bạn đang tìm kiếm một sân chơi lô đề an toàn, hấp dẫn và đổi thưởng nhanh chóng, đừng ngần ngại tham gia ngay hôm nay! Chắc chắn rằng bạn sẽ có những trải nghiệm thú vị và có cơ hội trúng thưởng lớn tại đây.
Câu lạc bộ bóng đá AS Roma
ST666 tự hào là đối tác tài trợ hàng đầu của câu lạc bộ bóng đá AS Roma – một biểu tượng vĩ đại trong làng bóng đá Ý. AS Roma không chỉ là đội bóng nổi tiếng với lịch sử thi đấu ấn tượng mà còn là đối tác quan trọng trong các hợp đồng hợp tác với ST666. Với sự hợp tác này, AS Roma đã hỗ trợ nhà cái ST666 thông qua những khoản đầu tư triệu đô, giúp phát triển mạnh mẽ mảng thể thao trực tuyến và mở ra cơ hội lớn cho người chơi cá cược tại thị trường quốc tế.
Câu lạc bộ bóng đá Bayern Munich
ST666 cũng vinh dự là nhà tài trợ hàng đầu của câu lạc bộ bóng đá Bayern Munich – một đội bóng nổi tiếng không chỉ ở Đức mà còn trên toàn thế giới. Với lịch sử vô địch lẫy lừng và sự thống trị tại các giải đấu quốc tế, Bayern Munich đã trở thành biểu tượng lớn trong lòng người hâm mộ. Sự hợp tác giữa ST666 và Bayern Munich không chỉ dừng lại ở việc tài trợ mà còn góp phần nâng cao trải nghiệm của người chơi thông qua cải tiến hệ thống giao dịch tài chính, giúp quy trình nạp rút tiền của bet thủ trở nên thuận tiện và nhanh chóng hơn.
Câu lạc bộ bóng đá Porto
Không thể không nhắc đến câu lạc bộ bóng đá Porto, nơi ST666 hân hạnh trở thành đối tác tài trợ hàng đầu. Porto không chỉ là biểu tượng của sự sáng tạo và thành công trong làng bóng đá Bồ Đào Nha mà còn hỗ trợ ST666 trong các thủ tục xin giấy phép và chứng nhận tại châu Âu và Philippines. Sự hợp tác này đã mang lại giá trị lớn cho ST666 khi mở rộng quy mô hoạt động ra nhiều khu vực và thị trường mới.
Câu lạc bộ bóng đá Arsenal
Trong làng bóng đá Anh, câu lạc bộ bóng đá Arsenal là biểu tượng của sự tinh tế và phong cách. ST666 rất tự hào khi trở thành nhà tài trợ chính của Arsenal, giúp nhà cái này không chỉ nâng cao vị thế mà còn mở rộng thị trường cá cược bóng đá toàn cầu. Arsenal đã đóng vai trò quan trọng trong việc hỗ trợ ST666 phát triển hệ thống cá cược, từ đó mang lại trải nghiệm tốt nhất cho người chơi.
Câu lạc bộ bóng đá Sevilla
Cuối cùng, câu lạc bộ bóng đá Sevilla – biểu tượng của sự khát vọng và thành công trong bóng đá Tây Ban Nha, cũng đã trở thành đối tác quan trọng của ST666. Sevilla không chỉ góp phần thúc đẩy ST666 về mặt thương mại mà còn cung cấp công nghệ bảo mật hàng đầu, đảm bảo rằng thông tin cá nhân, tài khoản và các giao dịch của người chơi đều được bảo vệ một cách tối ưu.
Điểm vượt trội của ST666 so với các đối thủ cạnh tranh khác
Sự hợp tác chiến lược giữa ST666 và các câu lạc bộ bóng đá hàng đầu không chỉ nâng cao vị thế của ST666 trên thị trường, mà còn mang lại những lợi ích vượt trội cho người chơi. Những điểm mạnh này bao gồm:
Hệ thống bảo mật tiên tiến: ST666 đã được Sevilla hỗ trợ trong việc nâng cấp công nghệ bảo mật, đảm bảo mọi giao dịch và thông tin người dùng đều an toàn.
Hệ thống nạp rút tiện lợi: Sự hợp tác với Bayern Munich giúp ST666 cải tiến hệ thống giao dịch tài chính, mang lại trải nghiệm nạp rút nhanh chóng và hiệu quả cho người chơi.
Mở rộng thị trường và dịch vụ: Nhờ sự hỗ trợ từ Arsenal và AS Roma, ST666 đã thành công trong việc mở rộng hệ thống cá cược bóng đá và tiếp cận thêm nhiều người chơi trên toàn thế giới.
ST666 không chỉ là một nhà cái hàng đầu mà còn là một đối tác đáng tin cậy của các câu lạc bộ bóng đá danh tiếng, luôn cam kết mang lại dịch vụ tốt nhất và trải nghiệm tối ưu cho người chơi.
nagano tonic: nagano tonic
קישורים מומלצים טלגרם כיוונים לקרית ים אמין
Перевод документов – это сложная и ответственная задача, требующая специальных знаний и опыта. В нашем бюро переводов работают профессиональные переводчики, которые гарантируют точность и качество перевода. Мы также оказываем услугу апостиля в Новосибирске, что позволяет легализовать документы за рубежом.
перевод с иностранных языков
перевод документов
augmentin 375mg without prescription – purchase levothyroxine online cheap brand levothyroxine
st666
Nhà cái ST666 được biết đến là sân chơi uy tín cung cấp các sản phẩm trò chơi cực kỳ đa dạng và chất lượng. Số lượng người tham gia vào hệ thống ngày càng tăng cao và chưa có dấu hiệu dừng lại. Hướng dẫn tham gia nhà cái nếu như anh em muốn tìm kiếm cơ hội nhận thưởng khủng. Hãy cùng khám phá những thông tin cần biết tại nhà cái để đặt cược và săn thưởng thành công.
Перевод документов: Как выбрать надежное бюро переводов для перевода важных документов? Апостиль в Новосибирске: Что такое апостиль и когда он нужен для легализации документов в другой стране?
перевод документов
sugar defender rviews: https://sugardefenderreviews.pages.dev
sugar defender rviews: https://sugardefenderreviews.pages.dev
sugar defender rviews: https://sugardefenderreviews.pages.dev
buy clindamycin for sale – cleocin tablet buy indocin pills for sale
https://cvzen.es/ CVzen es el servicio lider en redaccion de curriculums y coaching de carrera, confiado por millones de buscadores de empleo en todo el mundo. Nuestro equipo de expertos esta dedicado a tu exito, brindandote apoyo durante todo tu recorrido profesional. Desde la creacion de un curriculum profesional y una carta de presentacion adaptada a tu industria hasta la optimizacion de tu perfil de LinkedIn y el coaching de carrera personalizado, estamos aqui para ayudarte a desbloquear nuevas oportunidades con un curriculum que refleje tu verdadero potencial.
https://отель-судак.рф/
замена венцов
rgbet
Cách Tối Đa Hóa Tiền Thưởng Tại RGBET
META title: Cách tối ưu hóa tiền thưởng trên RGBET
META description: Học cách tối đa hóa tiền thưởng của bạn trên RGBET bằng các mẹo và chiến lược cá cược hiệu quả nhất.
Giới thiệu
Tiền thưởng là cơ hội tuyệt vời để tăng cơ hội thắng lớn mà không phải bỏ ra nhiều vốn. RGBET cung cấp rất nhiều loại khuyến mãi hấp dẫn, hãy cùng khám phá cách tối ưu hóa chúng!
1. Đọc kỹ điều kiện khuyến mãi
Mỗi chương trình thưởng đều có điều kiện riêng, bạn cần đọc kỹ để đảm bảo không bỏ lỡ cơ hội.
2. Đặt cược theo mức yêu cầu
Để nhận thưởng, hãy đảm bảo rằng số tiền cược của bạn đạt yêu cầu tối thiểu.
3. Tận dụng tiền thưởng nạp đầu tiên
Đây là cơ hội để bạn có thêm vốn ngay từ đầu. Hãy lựa chọn mức nạp phù hợp với ngân sách.
4. Theo dõi khuyến mãi hàng tuần
RGBET liên tục đưa ra các chương trình khuyến mãi hàng tuần. Đừng bỏ lỡ cơ hội nhận thêm tiền thưởng!
5. Sử dụng điểm VIP
Khi bạn đạt mức VIP, điểm thưởng có thể được chuyển thành tiền mặt, giúp bạn tối ưu hóa lợi nhuận.
Kết luận
Biết cách tận dụng tiền thưởng không chỉ giúp bạn có thêm tiền cược mà còn tăng cơ hội thắng lớn.
замена венцов
I’m in awe of the way you handle topics with both grace and authority.
order losartan pills – order cephalexin for sale cephalexin 125mg pills
The attention to detail is remarkable. I appreciate the thoroughness of The post.
order eurax without prescription – brand crotamiton order aczone online
В современном мире перевод с иностранных языков стал неотъемлемой частью нашей жизни. Мы ежедневно сталкиваемся с необходимостью перевода при общении в интернете, чтении зарубежной литературы, просмотре фильмов и сериалов, а также в бизнесе и образовании. Но как выбрать надежную языковую службу, которая гарантирует высокое качество перевода и соблюдение конфиденциальности?
Одним из ключевых факторов при выборе языковой службы является репутация компании. В Новосибирске работают как крупные переводческие агентства, так и небольшие бюро, только начинающие свой путь в этой сфере. Отзывы клиентов, опыт работы на рынке, наличие сертификатов и лицензий – все это поможет сделать правильный выбор.
Однако репутация – не единственный фактор, на который стоит обращать внимание. Важную роль играет также компетентность и опыт переводчиков. Перевод – это не просто механическое перенесение текста с одного языка на другой, это искусство, требующее глубокого знания языков, культуры и специфики терминологии в конкретной области. Поэтому так важно, чтобы переводчики имели специальное образование и опыт работы в данной сфере.
Кроме того, необходимо учитывать формат и объем перевода. Если вам нужен устный перевод, то понадобится синхронный или последовательный переводчик, который будет работать в режиме реального времени. Для письменных переводов может потребоваться предварительное изучение материала и использование специальных программ для работы с текстами.
Цена также является важным фактором при выборе языковой службы. Стоимость услуг может варьироваться в зависимости от языка, объема перевода, срочности и других факторов. Некоторые компании предлагают фиксированные цены за страницу или слово, другие – гибкую систему тарификации. Но не стоит гнаться за самой низкой ценой – она часто оборачивается низким качеством перевода.
Кроме того, необходимо учитывать необходимость нотариального заверения перевода. Если документ будет использоваться в официальных инстанциях, то он должен быть правильно оформлен и заверен нотариусом. Не все языковые службы предоставляют эту услугу, поэтому стоит заранее уточнить ее наличие.
Наконец, следует обратить внимание на такие нюансы, как сроки выполнения заказа и возможность доставки готового перевода. Если вам нужен срочный перевод, то стоит выбрать компанию, которая может гарантировать быструю обработку заказа. Также удобно, когда языковая служба предлагает доставку готовых переводов на дом или в офис.
В заключение можно сказать, что выбор языковой службы в Новосибирске – это ответственное решение, требующее тщательного подхода и анализа различных факторов. Но если подойти к процессу грамотно, то можно найти надежного партнера, который поможет преодолеть языковые барьеры и добиться успеха в бизнесе или в личной жизни. Главное – не экономить на качестве перевода, ведь правильный выбор языковой службы может стать залогом вашего успеха.
перевод с иностранных языков
ST666 – Nhà Cái Uy Tín Với Nhiều Khuyến Mãi Hấp Dẫn
ST666 là một trong những sòng bài trực tuyến uy tín nhất, mang đến cho các thành viên nhiều chương trình khuyến mãi đa dạng và hấp dẫn. Chúng tôi luôn hy vọng rằng những ưu đãi đặc biệt này sẽ mang lại trải nghiệm cá cược tuyệt vời cho tất cả thành viên của mình.
Khuyến Mãi Nạp Đầu Thưởng 100%
NO.1: Thưởng 100% lần nạp đầu tiên Thông thường, các khuyến mãi nạp tiền lần đầu đi kèm với yêu cầu vòng cược rất cao, thường từ 20 vòng trở lên. Tuy nhiên, ST666 hiểu rằng người chơi thường đắn đo khi quyết định nạp tiền vào sòng bài trực tuyến. Do đó, chúng tôi chỉ yêu cầu 8 vòng cược, cho phép người chơi rút tiền một cách nhanh chóng trong vòng 5 phút.
Thưởng 100% khi Đăng Nhập ST666
NO.2: Thưởng 16% mỗi ngày ST666 mang đến cho bạn một ưu đãi không thể bỏ lỡ. Mỗi khi bạn nạp tiền vào tài khoản hàng ngày, bạn sẽ được nhận thêm 16% số tiền nạp. Đây là cơ hội tuyệt vời dành cho những ai yêu thích cá cược trực tuyến và muốn gia tăng cơ hội chiến thắng của mình.
Bảo Hiểm Hoàn 10% Mỗi Ngày
NO.3: Bảo hiểm hoàn tiền 10% ST666 không chỉ hỗ trợ người chơi trong những lần may mắn mà còn luôn đồng hành khi bạn không gặp may. Chương trình khuyến mãi “bảo hiểm hoàn tiền 10% tổng tiền nạp mỗi ngày” là cách chúng tôi khích lệ và động viên tinh thần game thủ, giúp bạn tiếp tục hành trình chinh phục các thử thách cá cược.
Giới Thiệu Bạn Bè Thưởng 999K
NO.4: Giới thiệu bạn bè – Thưởng 999K Chương trình “Giới thiệu bạn bè” của ST666 giúp bạn có cơ hội cá cược cùng bạn bè và người thân. Không những vậy, bạn còn có thể nhận được phần thưởng lên đến 999K khi giới thiệu người bạn của mình tham gia ST666. Đây chính là một ưu đãi tuyệt vời giúp bạn vừa có thêm người đồng hành, vừa nhận được thưởng hấp dẫn.
Đăng Nhập Nhận Ngay 280K
NO.5: Điểm danh nhận thưởng 280K Nếu bạn là thành viên thường xuyên của ST666, hãy đừng quên nhấp vào hộp quà hàng ngày để điểm danh. Chỉ cần đăng nhập liên tục trong 7 ngày, cơ hội nhận thưởng 280K sẽ thuộc về bạn!
Kết Luận
ST666 luôn nỗ lực không ngừng để mang lại những chương trình khuyến mãi hấp dẫn và thiết thực nhất cho người chơi. Dù bạn là người mới hay đã gắn bó lâu năm với nền tảng, ST666 cam kết cung cấp trải nghiệm cá cược tốt nhất, an toàn và công bằng. Hãy tham gia ngay để không bỏ lỡ các cơ hội tuyệt vời từ ST666!
The creativity shines through, making me wonder what else you could do with such a vivid imagination.
The warmth and intelligence in The writing is as comforting as a cozy blanket on a cold night.
В современном глобализированном мире перевод играет ключевую роль в общении между людьми разных языков и культур. Переводчики служат мостом, соединяющим народы, позволяя им обмениваться информацией, идеями и культурными ценностями. Перевод – это не просто техническая задача, но настоящее искусство и наука, требующая глубокого понимания языков, культур, истории и контекста.
Виды перевода: от письменного к устному, от литературного к техническому
Существует множество видов перевода, каждый из которых имеет свои особенности и требует определенных навыков и подходов. Письменный перевод включает в себя перевод текстов, таких как книги, статьи, документы и веб-сайты. Устный перевод, с другой стороны, включает в себя перевод устной речи во время конференций, переговоров, презентаций и других событий. В устном переводе существует два основных подхода: синхронный и последовательный. Кроме того, перевод подразделяется на литературный, технический, медицинский, юридический и другие виды, каждый из которых требует специальных знаний и навыков.
Навыки и качество переводчика
Хороший переводчик должен обладать не только отличным знанием языков, но и глубоким пониманием культуры, истории и контекста. Он должен быть внимательным к деталям, креативным и иметь чувство языка и культуры. Переводчик должен уметь точно и аутентично передавать смысл оригинального текста, сохраняя его стиль и тон. Важную роль играют также коммуникативные навыки, способность работать в команде и соблюдать сроки.
Технологии в переводе: от CAT-инструментов к нейронным сетям
С развитием технологий появились новые инструменты для перевода, которые значительно облегчают работу переводчиков. CAT-инструменты (Computer-Assisted Translation) позволяют переводчикам работать быстрее и эффективнее, сохраняя стиль и терминологию оригинала. Машинный перевод, основанный на нейронных сетях, также стал полезным инструментом, особенно при переводах больших объемов текста. Однако, несмотря на все достижения в области технологий, человеческий фактор и творческий подход всегда останутся частью высококачественного перевода.
Вызовы и возможности в современной лингвистике
В современной лингвистике перевод стал объектом глубокого изучения. Одним из основных вызовов является сохранение культурной и эмоциональной окраски исходного текста при переводе. Это требует от переводчика не только знания языков, но и понимания контекста, культурных нюансов и тонкостей. Денции включают автоматизацию переводов с помощью искусственного интеллекта и разработку новых методов обучения переводчиков для более качественной работы.
Профессиональные возможности для переводчиков
Для переводчиков открывается широкий спектр профессиональных возможностей. Они могут работать в различных областях, таких как литература, техника, медицина, право и многие другие. Спрос на квалифицированных переводчиков в мире растет, что делает профессию востребованной и перспективной. Переводчики могут работать как фрилансеры, так и в переводческих агентствах, а также в компаниях, которые нуждаются в услугах перевода.
В заключение, можно сказать, что перевод – это настоящее искусство и наука, требующая глубокого понимания языков, культур, истории и контекста. Существует множество видов перевода, каждый из которых имеет свои особенности и требует определенных навыков и подходов. Технологии в переводе облегчают работу переводчиков, но человеческий фактор и творческий подход всегда останутся частью высококачественного перевода. Для переводчиков открывается широкий спектр профессиональных возможностей, и спрос на квалифицированных переводчиков в мире растет. Переводчики служат мостом между культурами, переводя не только слова, но и идеи, эмоции и контекст, способствуя взаимопониманию и сотрудничеству в глобальном мире.
перевод с иностранных языков
buy modafinil 100mg – purchase melatonin for sale how to get melatonin without a prescription
bupropion 150mg cheap – buy shuddha guggulu generic shuddha guggulu online
Joy to read and contagious enthusiasm? I thought I was immune, but you proved me wrong.
RGBET Trang Chủ Và Câu Chuyện Thương Hiệu
Ra đời vào năm 2010 tại Đài Loan, RGBET nhanh chóng trở thành một trang cá cược chất lượng hàng đầu khu vực Châu Á. Nhà cái được cấp phép hoạt động hợp pháp bởi công ty giải trí trực tuyến hợp pháp được ủy quyền và giám sát theo giấy phép Malta của Châu Âu – MGA. Và chịu sự giám sát chặt chẽ của tổ chức PAGCOR và BIV.
RGBET trang chủ cung cấp cho người chơi đa dạng các thể loại cược đặc sắc như: thể thao, đá gà, xổ số, nổ hũ, casino trực tuyến. Dịch vụ CSKH luôn hoạt động 24/7. Với chứng chỉ công nghệ GEOTRUST, nhà cái đảm bảo an toàn cho mọi giao dịch của khách hàng. APP RG thiết kế tối ưu giải quyết mọi vấn đề của người dùng IOS và Android.
Là một nhà cái đến từ đất nước công nghệ, nhà cái luôn không ngừng xây dựng và nâng cấp hệ thống game và dịch vụ hoàn hảo. Mọi giao dịch nạp rút được tự động hoá cho phép người chơi hoàn tất giao dịch chỉ với 2 phút vô cùng nhanh chóng
RGBET Lớn Nhất Uy Tín Nhất – Giá Trị Cốt Lõi
Nhà Cái RG Và Mục Tiêu Thương Hiệu
Giá trị cốt lõi mà RGBET mong muốn hướng đến đó chính là không ngừng hoàn thiện để đem đến một hệ thống chất lượng, công bằng và an toàn. Nâng cao sự hài lòng của người chơi, đẩy mạnh hoạt động chống gian lận và lừa đảo. RG luôn cung cấp hệ thống kèo nhà cái đặc sắc, cùng các sự kiện – giải đấu hàng đầu và tỷ lệ cược cạnh tranh đáp ứng mọi nhu cầu khách hàng.
Thương hiệu cá cược RGBET cam kết đem lại cho người chơi môi trường cá cược công bằng, văn minh và lành mạnh. Đây là nguồn động lực to lớn giúp nhà cái thực tế hóa các hoạt động của mình.
RGBET Có Tầm Nhìn Và Sứ Mệnh
Đổi mới và sáng tạo là yếu tố cốt lõi giúp đạt được mục tiêu dưới sự chuyển mình mạnh mẽ của công nghệ. Tầm nhìn và sứ mệnh của RGBET là luôn tìm tòi những điều mới lạ, đột phá mạnh mẽ, vượt khỏi giới hạn bản thân, đương đầu với thử thách để đem đến cho khách hàng sản phẩm hoàn thiện nhất.
Chúng tôi luôn sẵn sàng tiếp thu ý kiến và nâng cao bản thân mỗi ngày để tạo ra sân chơi bổ ích, uy tín và chuyên nghiệp cho người chơi. Để có thể trở thành nhà cái phù hợp với mọi khách hàng.
Khái Niệm Giá Trị Cốt Lõi Nhà Cái RGBET
Giá trị cốt lõi của nhà cái RG luôn gắn kết chặt chẽ với nhau giữa 5 khái niệm: Chính trực, chuyên nghiệp, an toàn, đổi mới, công nghệ.
Chính Trực
Mọi quy luật, cách thức của trò chơi đều được nhà cái cung cấp công khai, minh bạch và chi tiết. Mỗi tựa game hoạt động đều phải chịu sự giám sát kỹ lưỡng bởi các cơ quan tổ chức có tiếng về sự an toàn và minh bạch của nó.
Chuyên Nghiệp
Các hoạt động tại RGBET trang chủ luôn đề cao sự chuyên nghiệp lên hàng đầu. Từ giao diện đến chất lượng sản phẩm luôn được trau chuốt tỉ mỉ từng chi tiết. Thế giới giải trí được xây dựng theo văn hóa Châu Á, phù hợp với đại đa số thị phần khách Việt.
An Toàn
RG lớn nhất uy tín nhất luôn ưu tiên sử dụng công nghệ mã hóa hiện đại nhất để đảm bảo an toàn, riêng tư cho toàn bộ thông tin của người chơi. Đơn vị cam kết nói không với hành vi gian lận và mua bán, trao đổi thông tin cá nhân bất hợp pháp.
Đổi Mới
Nhà cái luôn theo dõi và bắt kịp xu hướng thời đại, liên tục bổ sung các sản phẩm mới, phương thức cá cược mới và các ưu đãi độc lạ, mang đến những trải nghiệm thú vị cho người chơi.
Công Nghệ
RGBET trang chủ tập trung xây dựng một giao diện game sắc nét, sống động cùng tốc độ tải nhanh chóng. Ứng dụng RGBET giải nén ít dung lượng phù hợp với mọi hệ điều hành và cấu hình, tăng khả năng sử dụng của khách hàng.
RGBET Khẳng Định Giá Trị Thương Hiệu
Hoạt động hợp pháp với đầy đủ giấy phép, chứng chỉ an toàn đạt tiêu chuẩn quốc tế
Hệ thống game đa màu sắc, đáp ứng được mọi nhu cầu người chơi
Chính sách bảo mật RG hiện đại và đảm bảo an toàn cho người chơi cá cược
Bắt tay hợp tác với nhiều đơn vị phát hành game uy tín, chất lượng thế giới
Giao dịch nạp rút RG cấp tốc, nhanh gọn, bảo mật an toàn
Kèo nhà cái đa dạng với bảng tỷ lệ kèo cao, hấp dẫn
Dịch Vụ RGBET Casino Online
Dịch vụ khách hàng
Đội ngũ CSKH RGBET luôn hoạt động thường trực 24/7. Nhân viên được đào tạo chuyên sâu luôn giải đáp tất cả các khó khăn của người chơi về các vấn đề tài khoản, khuyến mãi, giao dịch một cách nhanh chóng và chuẩn xác. Hạn chế tối đa làm ảnh hưởng đến quá trình trải nghiệm của khách hàng.
Đa dạng trò chơi
Với sự nhạy bén trong cập nhật xu thế, nhà cái RGBET đã dành nhiều thời gian phân tích nhu cầu khách hàng, đem đến một kho tàng game chất lượng với đa dạng thể loại từ RG casino online, thể thao, nổ hũ, game bài, đá gà, xổ số.
Khuyến mãi hấp dẫn
RGBET trang chủ liên tục cập nhật và thay đổi các sự kiện ưu đãi đầy hấp dẫn và độc đáo. Mọi thành viên bất kể là người chơi mới, người chơi cũ hay hội viên VIP đều có cơ hội được hưởng ưu đãi đặc biệt từ nhà cái.
Giao dịch linh hoạt, tốc độ
Thương hiệu RGBET luôn chú tâm đến hệ thống giao dịch. Nhà cái cung cấp dịch vụ nạp rút nhanh chóng với đa dạng phương thức như thẻ cào, ví điện tử, ngân hàng điện tử, ngân hàng trực tiếp. Mọi hoạt động đều được bảo mật tuyệt đối bởi công nghệ mã hóa tiên tiến.
App cá độ RGBET
App cá độ RGBET là một ứng dụng cho phép người chơi đăng nhập RG nhanh chóng, đồng thời các thao tác đăng ký RG trên app cũng được tối ưu và trở nên đơn giản hơn. Tham gia cá cược RG bằng app cá độ, người chơi sẽ có 1 trải nghiệm cá cược tuyệt vời và thú vị.
RGBET Có Chứng Nhận Cá Cược Quốc Tế
Nhà cái RGBET hoạt động hợp pháp dưới sự cấp phép của hai tổ chức thế giới là PAGCOR và MGA, tính minh bạch và công bằng luôn được giám sát gắt gao bởi BIV. Khi tham gia cược tại đây, người chơi sẽ được đảm bảo quyền và lợi ích hợp pháp của mình.
Việc sở hữu các chứng nhận quốc tế còn cho thấy nguồn tài chính ổn định, dồi dào của RGBET. Điều này cho thấy việc một nhà cái được công nhận bởi các cơ quan quốc tế không phải là một chuyện dễ.
Theo quy định nhà cái RGBET, chỉ người chơi từ đủ 18 tuổi trở lên mới có thể tham gia cá cược tại RGBET
MGA (Malta Gaming Authority)
Tổ chức MGA đảm bảo tính vẹn toàn và ổn định của các trò chơi. Có các chính sách bảo vệ nguồn tài chính và quyền lợi của người chơi. Chứng nhận một nhà cái hoạt động có đầy đủ pháp lý, tuân thủ nghiêm chỉnh luật cờ bạc.
Chứng nhận Quần đảo Virgin Vương quốc Anh (BIV)
Tổ chứng chứng nhận nhà cái có đầy đủ tài chính để hoạt động kinh doanh cá cược. Với nguồn ngân sách dồi dào, ổn định nhà cái bảo đảm tính thanh khoản cho người chơi, mọi quyền lợi sẽ không bị xâm phạm.
Giấy Phép PAGCOR
Tổ chức cấp giấy phép cho nhà cái hoạt động đạt chuẩn theo tiêu chuẩn quốc tế. Cho phép nhà cái tổ chức cá cược một cách hợp pháp, không bị rào cản. Có chính sách ngăn chặn mọi trò chơi có dấu hiệu lừa đảo, duy trì sự minh bạch, công bằng.
Nhà Cái RGBET Phát Triển Công Nghệ
Nhà cái RGBET hỗ trợ trên nhiều thiết bị : IOS, Android, APP, WEB, Html5
RG và Trách Nhiệm Xã Hội
RGBET RichGame không đơn thuần là một trang cá cược giải trí mà nhà cái còn thể hiện rõ tính trách nhiệm xã hội của mình. Đơn vị luôn mong muốn người chơi tham gia cá cược phải có trách nhiệm với bản thân, gia đình và cả xã hội. Mọi hoạt động diễn ra tại RGBET trang chủ nói riêng hay bất kỳ trang web khác, người chơi phải thật sự bình tĩnh và lý trí, đừng để bản thân rơi vào “cạm bẫy của cờ bạc”.
RGBET RichGame với chính sách nghiêm cấm mọi hành vi xâm phạm thông tin cá nhân và gian lận nhằm tạo ra một môi trường cá cược công bằng, lành mạnh. Nhà cái khuyến cáo mọi cá nhân chưa đủ 18 tuổi không nên đăng ký RG và tham gia vào bất kỳ hoạt động cá cược nào.
This post is a testament not only to The expertise but also to The dedication. Truly inspiring.
обратные ссылки купить
kubet
เว็บไซต์การพนันกีฬา KUBET เป็นแบรนด์ของไต้หวันภายใต้กลุ่ม Kyushu และเป็นแบรนด์การพนันออนไลน์ (เว็บเงินสด) ที่มีทุนสำรองมากกว่าพันล้าน มีอินเทอร์เฟซที่ใช้งานง่าย โปรโมชั่นมากมาย และกลุ่มเกมสำหรับสมาชิกซึ่งเป็นหนึ่งในคุณสมบัติเด่นของ KUBET กีฬา
KUBET กีฬาได้รับการอนุญาตและควบคุมโดยบริษัทความบันเทิงออนไลน์ที่ถูกกฎหมาย โดยมีใบอนุญาตจาก Malta Gaming Authority (MGA) ในยุโรปและใบอนุญาตจาก Philippine Amusement and Gaming Corporation (PAGCOR) จึงเป็นตัวเลือกแรกของผู้เล่นอย่างไม่ต้องสงสัย。
перевод документов
The posts are like stars in the sky—each one shining brightly, guiding my curiosity.
замена венцов
I look forward to The posts because they always offer something valuable. Another great read!
prometrium 100mg sale – buy ponstel online cheap buy clomiphene sale
Tullahoma Computer Repair
generic capecitabine – mefenamic acid drug danazol 100 mg over the counter
рюкзак подростковый для девочек черный
рюкзак женский непромокаемый
ранец для девочки
order norethindrone generic – cheap yasmin online how to buy yasmin
Когда наступает конец тяжёлого дня не можешь понять, как восстановить силы и избавиться от ежедневных забот? У нас имеется отличное решение! Этот ресурс — это площадка, в котором только для взрослых предлагаются самые разные наиболее увлекательные варианты для отдыха. Мы уверены, что любой наш пользователь каждый зашедший сумеет выбрать что-то особенное, что идеально подойдёт.
На этом сайте ты сможешь полностью окунуться в пространство наслаждений и радостей, где каждая мелочь продумана для твоего расслабления. Наши предложения разнообразны от любовных фильмов до интересных историй — лучший вариант для тех, кто ищет новые эмоции.
Не имеет значения, хочешь ли ты отдохнуть в одиночку или найти новые эмоции — эта площадка даст тебе идеальным досугом.
Мы отлично понимаем, насколько важно качественное расслабление, поэтому все развлечения на нашем сайте не просто велик, но и разработан с учётом всех нюансов. Отпусти мысли о работе и дай себе возможность насладиться в мир, где на первом месте удовольствие.
Наш портал создан для тех, кто любит себя, и мы с удовольствием дадим тебе то, что нужно для настоящего отдыха после сложной рабочей недели.
Только для зрелых. Лично для тебя. Заходи прямо сейчас и начни наслаждаться!
Superb postings. Appreciate it.
how to write a college narrative essay write essays for me construction dissertation
order dostinex pills – dostinex 0.25mg tablet buy alesse online cheap
線上德撲
buy yasmin no prescription – ginette 35 uk buy arimidex 1 mg generic
ST666 – Nhà Cái Uy Tín Hàng Đầu Việt Nam
ST666 là một trong những nhà cái uy tín số 1 tại Việt Nam, cung cấp đa dạng các trò chơi cá cược hấp dẫn như cá cược thể thao, casino, xổ số, đá gà, bắn cá, và nhiều trò chơi thú vị khác. Không chỉ vậy, ST666 còn mang đến các chương trình khuyến mãi hấp dẫn, giúp người chơi nhận được nhiều ưu đãi hấp dẫn khi tham gia.
Trò Chơi Cá Cược Tại ST666
ST666 cung cấp các trò chơi cá cược đa dạng, đáp ứng nhu cầu của mọi đối tượng người chơi. Các trò chơi nổi bật tại ST666 bao gồm:
Cá Cược Thể Thao: Các sự kiện thể thao hấp dẫn từ bóng đá, tennis đến các môn thể thao khác.
Casino: Trải nghiệm các trò chơi casino với chất lượng cao như blackjack, baccarat, roulette, và các trò chơi slot machines.
Đá Gà: Trận đấu đá gà gay cấn, nơi người chơi có thể tham gia cá cược và theo dõi những trận đấu kịch tính.
Xổ Số: Chơi xổ số và thử vận may để nhận những phần thưởng giá trị.
Bắn Cá: Trò chơi bắn cá với đồ họa sống động và hấp dẫn.
Esports: Tham gia cá cược vào các trận đấu thể thao điện tử hấp dẫn với các đội tuyển hàng đầu.
Khuyến Mãi Hấp Dẫn
ST666 luôn có các chương trình khuyến mãi hấp dẫn để tri ân người chơi, bao gồm:
Khuyến Mãi 100% Nạp Lần Đầu: Người chơi mới khi đăng ký tài khoản và nạp tiền lần đầu sẽ nhận được khuyến mãi nạp tiền lên tới 100%.
Bảo Hiểm Casino: ST666 cung cấp bảo hiểm cho các trò chơi casino, giúp người chơi giảm thiểu rủi ro khi tham gia.
Khuyến Mãi Thể Thao: Các chương trình khuyến mãi hấp dẫn dành riêng cho cá cược thể thao, giúp người chơi gia tăng cơ hội chiến thắng.
Đăng Ký và Đăng Nhập ST666
Để bắt đầu trải nghiệm các trò chơi và tham gia cá cược tại ST666, người chơi cần thực hiện các bước đơn giản sau:
Đăng Ký: Tạo tài khoản tại ST666 để nhận các ưu đãi và khuyến mãi hấp dẫn.
Đăng Nhập: Sau khi đăng ký thành công, người chơi có thể đăng nhập vào tài khoản để tham gia các trò chơi cá cược trực tuyến.
Nạp Tiền và Rút Tiền: ST666 hỗ trợ nhiều phương thức nạp và rút tiền linh hoạt, giúp người chơi giao dịch nhanh chóng và an toàn.
ST666 – Nơi Gửi Gắm Niềm Tin
ST666 không chỉ là nhà cái mà còn là nơi cung cấp môi trường cá cược công bằng và minh bạch. Người chơi luôn cảm thấy an tâm với các chính sách bảo mật chặt chẽ và dịch vụ hỗ trợ khách hàng 24/7. ST666 xứng đáng là điểm đến tin cậy cho những ai yêu thích cá cược trực tuyến.
Thông Tin Liên Hệ
Nếu bạn có bất kỳ câu hỏi hay thắc mắc nào, vui lòng liên hệ với ST666 qua các kênh hỗ trợ khách hàng. Chúng tôi luôn sẵn sàng giải đáp và hỗ trợ bạn.
CEO & Founder: Đặng Nhất Nam
Facebook ST666: Cập nhật những tin tức mới nhất và nhận các khuyến mãi hấp dẫn tại trang Facebook chính thức của ST666.
The analysis had the perfect mix of depth and clarity, like a perfectly mixed cocktail that I just can’t get enough of.
rtp slot
Judul: Mengalami Pengalaman Bertaruh dengan “PG Slot” di Situs Kasino ImgToon.com
Dalam kehidupan permainan kasino online, permainan slot telah menyusun salah satu permainan yang paling disukai, terutama jenis PG Slot. Di antara beberapa situs kasino online, ImgToon.com menjadi tujuan pokok bagi peserta yang ingin mencoba peruntungan mereka di beragam permainan slot, termasuk beberapa kategori terkenal seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Memulai Bebas dari Risiko
Salah satu fungsi menarik yang ditawarkan oleh ImgToon.com adalah demo pg slot. Keistimewaan ini memberikan pemain untuk menguji berbagai jenis slot dari PG tanpa harus menempatkan taruhan nyata. Dalam mode demo ini, Anda dapat menguji berbagai strategi dan mengerti proses permainan tanpa ancaman kehilangan uang. Ini adalah cara terbaik bagi pemain baru untuk terbiasa dengan permainan slot sebelum berpindah ke mode taruhan nyata.
Mode demo ini juga memberi Anda pemahaman tentang potensi kemenangan dan imbalan yang mungkin bisa Anda peroleh saat bermain dengan uang sebenarnya. Pemain dapat menyusuri permainan tanpa khawatir, menjadikan pengalaman bermain di PG Slot semakin mengasyikkan dan bebas tekanan.
PG Slot Gacor: Prospek Besar Mendulang Kemenangan
PG Slot Gacor adalah kata populer di kalangan pemain slot yang menggunakan pada slot yang sedang dalam fase memberi kemenangan tinggi atau lebih sering dikenal “gacor”. Di ImgToon.com, Anda dapat mencari berbagai slot yang termasuk dalam kategori gacor ini. Slot ini dikenal memiliki peluang kemenangan lebih tinggi dan sering menghadiahkan bonus besar, menyebabkannya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, penting diingat bahwa “gacor” atau tidaknya sebuah slot dapat berubah, karena permainan slot bergantung generator nomor acak (RNG). Dengan bermain secara rutin di ImgToon.com, Anda bisa mengenali pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan menambah peluang Anda untuk menang.
RTP Slot: Faktor Penting dalam Pemilahan Slot
Ketika mendiskusikan tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat esensial untuk dihitung. RTP Slot merujuk pada persentase dari total taruhan yang akan dipulangkan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot dilengkapi dengan informasi RTP yang jelas. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk memperoleh kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat mengoptimalkan pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini menjadikan RTP sebagai indikator utama bagi pemain yang mencari keuntungan dalam permainan kasino online.
Judul: Merasakan Pengalaman Bermain dengan “PG Slot” di Situs Kasino ImgToon.com
Dalam alam permainan kasino online, slot telah menjadi salah satu permainan yang paling digemari, terutama jenis PG Slot. Di antara banyak situs kasino online, ImgToon.com adalah tujuan terbesar bagi pemain yang ingin menguji nasib mereka di berbagai permainan slot, termasuk beberapa kategori terfavorit seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Memulai Tanpa adanya Risiko
Salah satu fungsi menarik yang diberikan oleh ImgToon.com adalah demo pg slot. Keistimewaan ini memberikan pemain untuk menguji berbagai jenis slot dari PG tanpa harus memasang taruhan sebenarnya. Dalam mode demo ini, Anda dapat memeriksa berbagai taktik dan mengetahui sistem permainan tanpa ancaman kehilangan uang. Ini adalah cara terbaik bagi pemula untuk beradaptasi dengan permainan slot sebelum mengalihkan ke mode taruhan nyata.
Mode demo ini juga memberikan Anda gambaran tentang potensi kemenangan dan imbalan yang mungkin bisa Anda dapatkan saat bermain dengan uang asli. Pemain dapat menyusuri permainan tanpa khawatir, menjadikan pengalaman bermain di PG Slot semakin membahagiakan dan bebas beban.
PG Slot Gacor: Kesempatan Besar Mendapatkan Kemenangan
PG Slot Gacor adalah istilah populer di kalangan pemain slot yang merujuk pada slot yang sedang dalam fase memberi kemenangan tinggi atau lebih sering dikenal “gacor”. Di ImgToon.com, Anda dapat mencari berbagai slot yang termasuk dalam kategori gacor ini. Slot ini terkenal memiliki peluang kemenangan lebih tinggi dan sering menghadiahkan bonus besar, menjadikannya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, penting diingat bahwa “gacor” atau tidaknya sebuah slot dapat beralih, karena permainan slot bergantung generator nomor acak (RNG). Dengan bermain secara rutin di ImgToon.com, Anda bisa menemukan pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan memperbesar peluang Anda untuk menang.
RTP Slot: Faktor Penting dalam Pencarian Slot
Ketika berbicara tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat penting untuk dipertimbangkan. RTP Slot berkaitan pada persentase dari total taruhan yang akan dikembalikan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot dilengkapi dengan informasi RTP yang terang. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk mendulang kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat mengoptimalkan pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini membuat RTP sebagai indikator penting bagi pemain yang mencari keuntungan dalam permainan kasino online.
Each post is a window into The thoughts, and I must say, the view is stunning.
Microblading Austin
Genuinely impressed by The analysis. I was starting to think depth had gone out of style. Kudos for proving me wrong!
Packed with insights, or what I call, a buffet for the brain.
You’ve done a fantastic job of breaking down this topic, like unlocking a door to a secret garden. Intrigued to explore more.
The depth you bring to The topics is like diving into a deep pool, refreshing and invigorating.
Explore a Universe of Virtual Opportunities with Items4Players
At Items4Games, we provide a active hub for players to purchase or exchange profiles, items, and services for some of the most popular video games. Whether you are seeking to upgrade your gaming inventory or wanting to profit from your profile, our site offers a seamless, protected, and rewarding experience.
Reasons to Choose Items4Play?
**Comprehensive Title Library**: Browse a extensive variety of titles, from thrilling adventures like Warzone and War Call to immersive RPGs like ARK and Genshin. We include it all, ensuring no enthusiast is left behind.
**Variety of Features**: Our products include game account acquisitions, in-game currency, exclusive items, milestones, and training services. Whether you want assistance gaining levels or obtaining exclusive rewards, we have it all.
**User-friendly**: Navigate without hassle through our structured platform, categorized alphabetically to find exactly the item you are looking for with ease.
**Secure Transactions**: We prioritize your protection. All trades on our site are handled with the utmost safeguarding to protect your confidential and monetary information.
**Highlights from Our Collection**
– **Adventure and Exploration**: Titles ARK: Survival Evolved and DayZ give you the chance to explore exciting worlds with premium items and keys on offer.
– **Tactical and Questing**: Boost your experience in adventures such as Clash Royale and Wonders Age with in-game currencies and services.
– **Competitive Gaming**: For serious fans, boost your technique with coaching and profile boosts for Valorant, DotA, and Legends.
**A Platform Designed for Fans**
Operated by ApexTech Innovations, a reliable entity officially recognized in Kazakh Nation, Items4Play is a market where gaming dreams are realized. From buying pre-order keys for the freshest releases to getting rare game items, our site caters to every player’s requirement with professionalism and reliability.
Join the community right away and boost your game adventure!
For support or guidance, reach out to us at **support@items4games.com**. Together, let’s game better, together!