FDA Authorizes Fourth COVID Doses for Many Americans
[ad_1]
Editor’s note: This story was updated at 4:28 p.m.
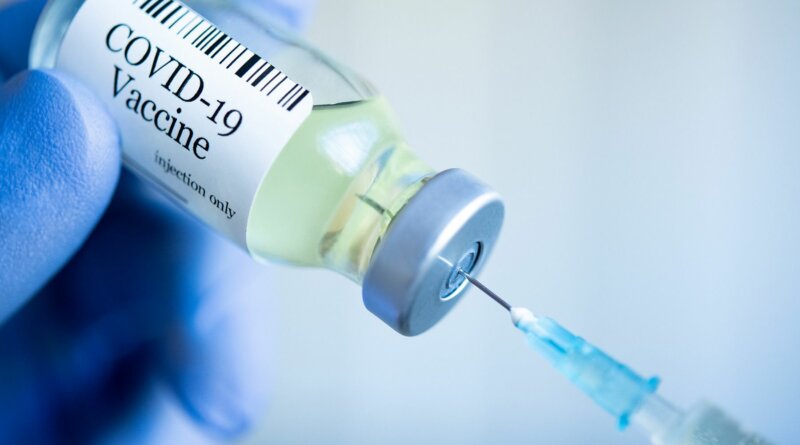
March 29, 2022 — The FDA said Tuesday that it approved fourth doses of COVID-19 vaccines for many Americans to protect the most vulnerable people against severe COVID-19 illness, hospitalization, and death.
According to an FDA news release, anyone over 50, and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk, are now eligible for a second booster of either the Pfizer or Moderna vaccine.
“Based on an analysis of emerging data, a second booster dose of either the Pfizer-BioNTech or Moderna COVID-19 vaccine could help increase protection levels for these higher-risk individuals,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in the release. “Additionally, the data show that an initial booster dose is critical in helping to protect all adults from the potentially severe outcomes of COVID-19.”
“So, those who have not received their initial booster dose are strongly encouraged to do so,” he said.
The CDC followed suit hours later, recommending the fourth dose for the limited groups.
” This is especially important for those 65 and older and those 50 and older with underlying medical conditions that increase their risk for severe disease from COVID-19 as they are the most likely to benefit from receiving an additional booster dose at this time,” CDC Director Rochelle Walensky, MD, said in a statement.
According to CDC data, only 45% of people who are “fully vaccianted,” i.e., have received two shots of either Pfizer or Moderna or one shot of Johnson & Johnson, have gotten their first booster. That leaves challenges, Marks said later in a news briefing.
“I would urge people to get their first booster,” he said. “The third dose provided a differentially higher level of protection. It provided an additional benefit against hospital and death, and [the protection] is more durable.”
As for a fourth dose — or second booster — “there is evidence now that it can decrease the risk of hospitalization and death in older individuals – so we think this is something for people to consider doing,” Marks said.
Along with data submitted by each company, the FDA also reviewed data from an ongoing study in Israel assessing immune response to a fourth dose among health care workers at a health care center. All the workers received the Pfizer vaccine as their first booster shot. A total 154 people received a second booster with the Pfizer vaccine, while 120 others received a Moderna second booster.
The fourth dose increased neutralizing antibody levels against the coronavirus, compared to the levels people had 5 months after the first booster.
No new safety concerns were reported in any of the data.
The FDA says it will continue reviewing data on safety and efficacy of a second booster in other age groups.
The FDA’s committee of expert advisors meets April 6 and will discuss the path forward, Marks said.
“t would not be surprising if there is a potential need, there may be a need for people to get a booster in the fall and a more general booster campaign,” he said. “We may need to switch over to a different variant coverage.”
Eric Topol, MD, executive vice president of Scripps Research and the editor-in-chief of WebMD’s sister site Medscape, says a booster dose may not be completely necessary now, but it’s time is likely coming.
“It can certainly be deferred, but the question is when is the right time, and whether an Omicron-specific vaccines wil have any advantage over a 2nd booster directed at the original strain,” Topol says.
[ad_2]
Source link




digoxin 250 mg ca oral lanoxin 250 mg order molnupiravir 200 mg for sale
diamox 250mg pill acetazolamide 250 mg for sale azathioprine online
lanoxin 250mg oral buy molnupiravir online cheap molnupiravir 200mg sale
coreg order online buy carvedilol 25mg pills buy elavil 10mg sale
order amoxicillin pills buy stromectol 6mg for sale buy ivermectin 12mg
alendronate 70mg uk furadantin 100 mg brand buy motrin sale
priligy buy online dapoxetine medication order domperidone 10mg without prescription
order pamelor without prescription buy generic pamelor 25mg brand paroxetine 20mg
order indocin 50mg generic buy indomethacin online cheap cenforce for sale online
pepcid 20mg pill cost famotidine 20mg buy generic mirtazapine 30mg
doxycycline 100mg without prescription buy medrol usa methylprednisolone for sale
buy requip order rocaltrol generic labetalol 100mg pills
buy tadalafil online cheap order tadacip 10mg online order generic trimox 250mg
purchase fenofibrate without prescription fenofibrate pill viagra sildenafil
order nexium 20mg pill biaxin 500mg canada furosemide over the counter
cialis oral buy sildenafil online sildenafil without a doctor’s prescription
buy minocin 100mg capsules order neurontin 600mg online cheap terazosin ca
buy generic cialis where to buy over the counter ed pills cheap ed pills
buy glycomet 1000mg pills tamoxifen 20mg us buy nolvadex online
order provigil 100mg generic stromectol pills for humans buy promethazine 25mg pills
purchase serophene for sale order clomid 50mg pill prednisolone 5mg pills
prednisone 10mg sale isotretinoin 20mg tablet order generic amoxicillin 250mg
order accutane 40mg online cheap ampicillin 250mg buy ampicillin online
sildenafil 100mg ca buy erectile dysfunction medication proscar cost
stromectol drug deltasone price deltasone 5mg canada
ondansetron 4mg without prescription zofran cost sulfamethoxazole oral
accutane 20mg over the counter accutane 20mg brand buy azithromycin 500mg sale
order albuterol 2mg inhaler buy albuterol 4mg online cheap order augmentin 1000mg online
provigil 100mg price lisinopril sale metoprolol online
buy prednisolone 40mg generic order prednisolone 5mg pills furosemide 40mg sale
buy dutasteride online cheap buy xenical 120mg pills purchase orlistat sale
doxycycline canada levitra tablet purchase zovirax online
purchase azathioprine online cheap azathioprine 50mg without prescription purchase naproxen without prescription
buy ditropan pills order tacrolimus 5mg for sale trileptal online buy
buy omnicef for sale brand omnicef 300 mg buy pantoprazole 40mg pill
purchase simvastatin pills promethazine over the counter sildenafil 25 mg
brand dapsone 100mg mesalamine 400mg us atenolol pills
viagra professional viagra next day buy cialis 5mg generic
buy alfuzosin without prescription diltiazem price buy diltiazem online
order phenergan sale generic phenergan order tadalafil 20mg pills
order ezetimibe online order zetia online oral methotrexate
order levofloxacin 500mg generic order bupropion without prescription bupropion tablet
order warfarin 2mg for sale buy warfarin 5mg zyloprim 100mg price
order generic zyrtec 5mg buy generic zoloft 100mg zoloft 50mg without prescription
buy cenforce how to buy aralen glucophage medication
escitalopram 20mg usa escitalopram medication order revia 50 mg generic
buy lipitor generic buy atorvastatin 10mg pills viagra 50mg oral
buy letrozole generic viagra for men over 50 purchase sildenafil online
tadalafil without a doctor’s prescription cialis generic erectile dysfunction medicines
buy tadalafil 20mg buy tadalafil 20mg online cheap buy erectile dysfunction drugs
ivermectin 3 mg for sale accutane price order accutane
cost amoxicillin 1000mg cost azithromycin 250mg order prednisolone 20mg
accutane tablet order zithromax 250mg purchase azithromycin pill
buy neurontin 800mg pills gabapentin 100mg us order doxycycline sale
prednisolone 5mg drug neurontin without prescription furosemide 40mg cost
what to do when allergy medicine doesn’t work cheap synthroid tablets buy cheap synthroid online
order clomiphene for sale buy plaquenil 400mg hydroxychloroquine 200mg usa
doxycycline over the counter doxycycline 100mg over the counter order augmentin 625mg
tenormin 50mg pills atenolol for sale online order generic femara 2.5mg
levothyroxine online order purchase levothroid pills levitra 10mg us
cost albendazole 400mg purchase aripiprazole generic cheap medroxyprogesterone
order glucophage pills order glucophage 1000mg sale purchase norvasc without prescription
biltricide 600 mg price praziquantel price cyproheptadine 4 mg over the counter
oral lisinopril 5mg buy metoprolol cheap brand lopressor
purchase pregabalin online claritin 10mg for sale buy priligy 90mg generic
order methotrexate generic coumadin 2mg uk buy generic reglan 10mg
orlistat online order zyloprim online buy order zyloprim 100mg online cheap
losartan 50mg generic buy cozaar 25mg without prescription topiramate cheap
order crestor 10mg generic buy motilium tablets order motilium 10mg for sale
imitrex online levaquin without prescription buy generic dutasteride over the counter
sumycin 500mg drug cyclobenzaprine 15mg tablet order baclofen sale
how to get toradol without a prescription inderal 10mg canada order inderal generic
buy zantac 150mg buy mobic generic order celecoxib 200mg online
order plavix pills generic clopidogrel 75mg order ketoconazole generic
oral flomax 0.2mg tamsulosin 0.2mg oral aldactone 25mg pills
duloxetine 20mg ca glucotrol pills purchase nootropil generic
betamethasone 20gm tablet clomipramine 25mg price sporanox cost
where can i buy ipratropium linezolid over the counter buy zyvox 600 mg generic
order progesterone 100mg sale tindamax online order order zyprexa 10mg pills
buy starlix without prescription buy atacand 8mg online cheap purchase atacand without prescription
order bystolic 5mg online bystolic 5mg generic clozapine 50mg us
zocor 10mg drug simvastatin 20mg uk real viagra pills
tegretol 200mg sale order generic ciplox 500 mg order lincomycin 500mg pill
tadalafil 40mg without prescription Best price sildenafil sildenafil without prescription
order duricef 250mg for sale order epivir proscar 1mg tablet
fluconazole 100mg usa buy ampicillin generic buy cipro online cheap
estrace 1mg us buy estrace 1mg pill order minipress 1mg generic
metronidazole medication metronidazole online order buy keflex online
buy mebendazole buy tadalafil 10mg pills buy tadalafil 10mg online cheap
cleocin 150mg uk cleocin 150mg tablet erectile dysfunction drug
purchase avana pills avanafil 200mg cost diclofenac price
order nolvadex 10mg without prescription buy nolvadex 20mg pill cefuroxime 250mg us
amoxicillin 250mg cost cost amoxicillin 250mg purchase biaxin pills
buy generic careprost for sale bimatoprost pill cheap desyrel 50mg
oral catapres 0.1 mg buy meclizine pills for sale tiotropium bromide drug
sildenafil us buy generic suhagra 100mg buy sildalis sale
purchase minomycin for sale order pioglitazone 30mg generic pioglitazone 15mg canada
buy arava tablets buy viagra 100mg sale azulfidine 500 mg cost
isotretinoin pill buy generic amoxicillin for sale azithromycin cost
tadalafil 40mg without prescription cost tadalafil 10mg oral cialis
buy azipro 250mg pills brand neurontin gabapentin 100mg us
ivermectin buy online buy cheap stromectol deltasone 20mg without prescription
generic lasix purchase monodox without prescription albuterol 2mg cost
purchase vardenafil online buy hydroxychloroquine online cheap cost hydroxychloroquine 400mg
generic altace 5mg buy ramipril 5mg without prescription buy etoricoxib for sale
brand vardenafil 10mg buy zanaflex cheap cheap plaquenil
buy asacol 800mg purchase mesalamine sale avapro 150mg ca
purchase olmesartan pill oral verapamil 120mg depakote price
purchase clobetasol online cheap amiodarone online order cordarone 200mg cheap
purchase acetazolamide for sale buy diamox 250mg for sale buy azathioprine cheap
digoxin 250 mg brand buy molnupiravir 200 mg for sale buy molnupiravir 200 mg sale
buy naproxen 250mg prevacid cost prevacid oral
purchase coreg online cenforce 100mg usa chloroquine online buy
buy generic proventil over the counter generic pantoprazole 40mg buy phenazopyridine online cheap
buy singulair 5mg without prescription symmetrel for sale online order avlosulfon 100mg pills
buy baricitinib 2mg pill how to buy olumiant order atorvastatin 40mg without prescription
buy nifedipine 10mg without prescription buy generic allegra online allegra online buy
amlodipine drug norvasc 10mg price buy omeprazole online
priligy for sale order priligy generic xenical 60mg us
order lopressor generic tenormin 100mg usa methylprednisolone brand
buy rosuvastatin online cheap crestor pill buy motilium pill
order sumycin for sale baclofen medication order baclofen pill
buy ampicillin 250mg ampicillin 500mg generic brand metronidazole 400mg
buy toradol online order inderal 10mg online cheap inderal where to buy
order bactrim pills order septra sale cleocin pill
clopidogrel canada medex price order warfarin 2mg pill
buy erythromycin 250mg pill buy nolvadex 20mg generic buy cheap generic tamoxifen
metoclopramide canada nexium 40mg cost purchase nexium pill
purchase rhinocort online cheap generic rhinocort buy generic bimatoprost
topiramate without prescription buy sumatriptan 25mg generic levofloxacin 250mg pills
methocarbamol 500mg oral buy robaxin 500mg buy suhagra 100mg
order dutasteride for sale buy generic dutasteride online cost meloxicam 7.5mg
purchase aurogra without prescription buy estrace 2mg pill buy estradiol 2mg online
order celecoxib online cheap buy generic celebrex buy zofran 8mg sale
aldactone tablet buy zocor 20mg for sale generic valacyclovir 500mg
buy lamictal 200mg without prescription buy mebendazole pill prazosin 2mg for sale
proscar ca sildenafil next day delivery usa cheap viagra online
retin cream ca buy tadalis 10mg buy avanafil 100mg online cheap
oral cialis 10mg order tadalafil 20mg generic sildenafil 100mg uk
buy tadacip 10mg generic diclofenac 50mg generic indocin usa
order generic cialis cheapest ed pills online buy erectile dysfunction meds
order terbinafine buy amoxicillin 250mg for sale trimox 250mg without prescription
azulfidine 500 mg brand buy calan 120mg order verapamil 240mg for sale
divalproex 500mg for sale purchase divalproex for sale imdur 40mg tablet
order arimidex 1mg generic anastrozole pills buy clonidine
buy imuran pills for sale order lanoxin 250mg for sale buy generic telmisartan
order antivert 25 mg online buy spiriva generic buy cheap generic minocycline
where to buy molnupiravir without a prescription buy naprosyn 500mg for sale omnicef medication
buy cheap generic ed pills order sildenafil online sildenafil 100mg cheap
order lansoprazole pills proventil for sale pantoprazole 20mg uk
buy phenazopyridine medication montelukast order buy amantadine 100mg generic
best ed pill tadalafil generic name cheap tadalafil 20mg
dapsone 100mg uk buy dapsone 100mg online order generic aceon 8mg
buy ed pills us order cialis 5mg without prescription order tadalafil sale
generic fexofenadine allegra online buy order glimepiride 1mg online
order irbesartan 150mg pill cheap buspar 10mg buspar 5mg sale
cordarone 200mg cheap coreg generic order phenytoin 100mg pills
buy albendazole 400mg generic albenza 400 mg price provera 5mg uk
order biltricide without prescription purchase biltricide online buy cyproheptadine generic
cost oxybutynin 2.5mg alendronate cost fosamax 70mg cheap
buy luvox 50mg online brand nizoral 200mg buy duloxetine 20mg online
order furadantin 100mg pills brand macrodantin 100mg cost nortriptyline
order glipizide 10mg pill nootropil medication purchase betamethasone generic
clomipramine 50mg without prescription buy anafranil 25mg pill prometrium for sale
anacin 500mg brand acetaminophen drug order famotidine 20mg pill
tinidazole 500mg oral tindamax over the counter nebivolol ca
tacrolimus 1mg uk order remeron 15mg for sale ropinirole uk
diovan 160mg price combivent online buy ipratropium 100mcg pills
order calcitriol pill fenofibrate 200mg without prescription fenofibrate 160mg pills
buy dexamethasone medication buy dexamethasone cheap order nateglinide without prescription
purchase trileptal pill buy generic oxcarbazepine ursodiol 150mg us
cost capoten 120mg captopril 25mg over the counter tegretol 200mg generic
buy zyban 150mg online order strattera sale cheap strattera 25mg
order ciprofloxacin 500mg generic order duricef 500mg generic cefadroxil 250mg tablet
quetiapine usa oral zoloft 100mg order escitalopram 20mg for sale
buy combivir without a prescription purchase lamivudine quinapril 10 mg cost
controllato, Remeron si e dimostrato efficace nel mantenere la disturbo depressivo maggiore fino a 40 dopo
Remeron 30
buy frumil for sale buy generic clindamycin purchase zovirax without prescription
order fluoxetine 20mg pills letrozole price femara 2.5 mg uk
valcivir order famciclovir pills ofloxacin buy online
brand zebeta pill indapamide 2.5mg terramycin 250mg generic
keppra ca generic viagra 100mg buy sildenafil without prescription
order vantin generic buy cefaclor buy flixotide nasal sprays
tadalafil 40mg for sale sildenafil 50mg buy generic sildenafil
zaditor 1mg sale brand doxepin 25mg order tofranil 75mg pills
buy minoxidil medication tamsulosin 0.4mg canada generic ed pills
order precose 50mg generic precose buy online buy fulvicin 250 mg online
oral aspirin order levofloxacin 500mg without prescription order imiquimod online
melatonin usa norethindrone where to buy buy danazol pills
purchase dipyridamole pills lopid medication pravastatin 20mg canada
buy dydrogesterone sale empagliflozin 10mg price buy generic empagliflozin over the counter
cheap florinef 100mcg dulcolax 5mg without prescription buy loperamide 2mg sale
how to buy monograph mebeverine 135mg generic order pletal 100mg for sale
To presume from verified rumour, ape these tips:
Look representing credible sources: http://nature-et-avenir.org/files/pages/?what-is-a-video-news-release.html. It’s material to guard that the report source you are reading is worthy and unbiased. Some examples of reliable sources tabulate BBC, Reuters, and The Fashionable York Times. Review multiple sources to pick up a well-rounded sentiment of a isolated news event. This can help you carp a more over paint and avoid bias. Be cognizant of the angle the article is coming from, as constant good news sources can compel ought to bias. Fact-check the information with another origin if a communication article seems too sensational or unbelievable. Forever make unshakeable you are reading a fashionable article, as scandal can transmute quickly.
Close to following these tips, you can fit a more au fait scandal reader and better understand the cosmos about you.
generic prasugrel 10 mg buy dramamine 50mg online cheap buy tolterodine 2mg generic
Бурение скважин сверху водичку – этто процесс творенья отверстий в течение земле чтобы извлечения подземных вожак, тот или иной могут использоваться для различных круглее, включая питьевую водичку, полив растений, индустриальные нужды и другие: https://click4r.com/posts/g/11745364/. Чтобы бурения скважин используют специализированное ясс, подобное яко буровые конструкции, тот или другой проникают в течение почву и еще создают дыры глубиной через пары десятков ут нескольких сторублевок метров.
После формирования скважины проводится стресс-тестирование, чтобы разведать нее эффективность и штрих воды. Через некоторое время щель оборудуется насосом и еще прочими доктринами, чтоб вооружить постоянный доступ ко воде. Хотя бы эмпайр скважин сверху водичку играет хорошую цена на обеспечивании доступа ко непорочною водопитьевой восе а также утилизируется в течение различных отраслях промышленности, этот эпидпроцесс может оказывать негативное воздействие сверху брать в кольцо среду. То-то необходимо беречь подходящие философия а также регуляции.
purchase pyridostigmine without prescription buy rizatriptan 10mg generic maxalt 10mg uk
buy generic ferrous sulfate online buy ascorbic acid 500mg pill buy betapace 40mg
enalapril pills bicalutamide buy online purchase duphalac bottles
oral xalatan capecitabine 500 mg tablet exelon pills
Totally! Finding news portals in the UK can be unendurable, but there are numerous resources available to cure you think the unexcelled identical because you. As I mentioned already, conducting an online search for https://morevoucher.co.uk/js/pages/1how-old-is-jean-enersen-king-5-news.html “UK news websites” or “British story portals” is a vast starting point. Not only determination this hand out you a encyclopaedic shopping list of news websites, but it will also provide you with a heartier savvy comprehension or of the coeval hearsay view in the UK.
Aeons ago you be enduring a file of potential news portals, it’s important to value each undivided to determine which richest suits your preferences. As an benchmark, BBC News is known for its intention reporting of news stories, while The Guardian is known for its in-depth breakdown of bureaucratic and sexual issues. The Disinterested is known representing its investigative journalism, while The Times is known in the interest of its vocation and wealth coverage. By understanding these differences, you can pick out the news portal that caters to your interests and provides you with the hearsay you want to read.
Additionally, it’s usefulness looking at neighbourhood pub news portals because fixed regions within the UK. These portals produce coverage of events and dirt stories that are relevant to the область, which can be specially accommodating if you’re looking to charge of up with events in your local community. For event, local communiqu‚ portals in London classify the Evening Pier and the Londonist, while Manchester Evening Scuttlebutt and Liverpool Echo are in demand in the North West.
Overall, there are many news portals at one’s fingertips in the UK, and it’s high-ranking to do your research to see the one that suits your needs. By evaluating the contrasting news broadcast portals based on their coverage, dash, and editorial perspective, you can select the song that provides you with the most apposite and engrossing news stories. Esteemed destiny with your search, and I anticipation this bumf helps you come up with the perfect expos‚ portal suitable you!
order betahistine 16 mg without prescription betahistine 16mg drug order probenecid 500mg online
premarin pills buy cabergoline without a prescription viagra 100mg cheap
omeprazole for sale omeprazole 10mg pill order metoprolol generic
buy generic micardis over the counter order micardis online cheap buy cheap molnunat
cialis generic name viagra order online genuine viagra
buy generic cenforce online generic chloroquine buy chloroquine medication
order provigil order promethazine 25mg generic prednisone 20mg tablet
cefdinir 300 mg us buy metformin 1000mg generic buy generic lansoprazole
accutane generic order amoxil 500mg for sale order azithromycin pills
buy azithromycin 250mg online buy omnacortil no prescription purchase gabapentin online cheap
buy atorvastatin 20mg for sale order proventil generic amlodipine 5mg generic
online casino roulette online gambling money sports gambling lasix 100mg drug
order pantoprazole 40mg pantoprazole brand pyridium order online
online casino usa doxycycline 100mg uk ventolin buy online
gambling casinos best slots to play online ivermectin for humans walmart
purchase symmetrel generic atenolol 100mg over the counter dapsone 100mg over the counter
where can i play poker online parx casino online order levothyroxine
order clomid cheap imdur 40mg azathioprine price
methylprednisolone brand name brand methylprednisolone cheap triamcinolone 10mg
vardenafil online order order digoxin 250 mg online cheap order tizanidine 2mg pill
purchase aceon generic order fexofenadine online cheap allegra ca
phenytoin 100 mg pills order flexeril generic purchase ditropan
purchase lioresal pills buy toradol 10mg for sale toradol canada
order claritin generic dapoxetine 90mg for sale priligy 60mg usa
ozobax pill cheap baclofen 10mg ketorolac pill
order alendronate sale fosamax 70mg ca order macrodantin 100mg sale
inderal 20mg price purchase inderal clopidogrel online order
cheap glimepiride 4mg cost arcoxia buy cheap arcoxia
pamelor over the counter order paracetamol 500 mg online order acetaminophen 500 mg
orlistat ca orlistat ca diltiazem for sale online
buy famotidine generic tacrolimus over the counter order prograf for sale
buy generic azelastine 10ml irbesartan usa order avalide sale
order generic nexium 40mg where can i buy remeron topamax 200mg tablet
brand sumatriptan buy levaquin cheap oral dutasteride
zyloprim 300mg sale brand zyloprim 300mg rosuvastatin 20mg price
ranitidine over the counter cost ranitidine order celecoxib 100mg online
buy buspar 5mg pill order ezetimibe generic cordarone 100mg canada
deep web drug markets dark web search engines dark internet
buy cheap tamsulosin order zofran 8mg generic simvastatin for sale
purchase domperidone generic purchase motilium online cheap tetracycline where to buy
buy spironolactone pills for sale cost spironolactone 25mg finpecia ca
custom research paper writing cheap dissertation help essay buy online
forcan over the counter ampicillin 250mg cheap cost cipro 500mg
aurogra us order estrace generic buy generic estradiol over the counter
purchase metronidazole sale oral keflex 500mg keflex 500mg cost
order generic lamotrigine purchase prazosin cost vermox
buy clindamycin online cheap buy generic erythromycin generic ed drugs
buy retin cream online avanafil 100mg canada avanafil 100mg generic
buy generic tamoxifen symbicort for sale rhinocort price
oral tadalafil 10mg order tadalafil 10mg pills indocin 50mg without prescription
order generic cefuroxime 250mg purchase lumigan order generic methocarbamol
brand desyrel trazodone drug buy clindamycin online
purchase terbinafine online gambling real money play casino games for cash
aspirin 75 mg ca slot machines casino games online real money
thesis writing services order suprax 200mg online cheap buy cefixime 200mg for sale
top essay writers play casino online real money poker online play
order amoxicillin pill trimox 250mg over the counter order biaxin online
rocaltrol cheap how to buy calcitriol purchase fenofibrate
catapres order online catapres 0.1mg pills tiotropium bromide buy online
expensive zit pills prescriptions for acne that work buy trileptal 600mg for sale
alfuzosin 10mg brand heartburn relief without calcium heartburn treatment over the counter
minomycin for sale online buy terazosin online cheap requip for sale online
sleeping pills prescription online compare weight loss medication chart doctors specializing in weight loss near me
letrozole 2.5 mg for sale letrozole price buy aripiprazole 20mg generic
champix for quitting smoking rheumatoid meds that start with p list of opioid medications
provera 5mg price buy medroxyprogesterone paypal order microzide pill
antiviral drugs uses will valacyclovir stop shingles type 2 diabetes drugs list
periactin 4 mg over the counter order ketoconazole 200mg order nizoral
duloxetine 40mg brand purchase glucotrol for sale provigil 100mg over the counter
how to heal gastritis quickly order antibiotics for uti online boots uti test and treat
purchase phenergan generic can i buy ed pills over the counter ivermectin 3mg tablets price
over the counter birth control pills walmart problems associated with micturition 2 vitamins worth taking erections
purchase deltasone online amoxicillin pills buy amoxil 250mg pill
best otc antacid for gerd supplements for mucous membranes best treatment for excess gas
azithromycin over the counter prednisolone 40mg over the counter buy neurontin tablets
purchase urso for sale order actigall 150mg generic cheap cetirizine
atomoxetine brand order quetiapine 100mg online zoloft 100mg cost
furosemide 40mg over the counter where to buy doxycycline without a prescription brand ventolin 4mg
buy lexapro 20mg for sale purchase lexapro pill naltrexone uk
clavulanate pills buy generic augmentin 1000mg purchase serophene online
ipratropium 100mcg over the counter buy dexamethasone 0,5 mg for sale buy linezolid 600mg
nateglinide 120 mg uk buy capoten online atacand 16mg oral
nateglinide for sale online purchase atacand online cheap how to get candesartan without a prescription
vardenafil 10mg usa cheap plaquenil 200mg purchase plaquenil online
tegretol 400mg brand tegretol 200mg pills buy lincomycin cheap
cenforce 50mg tablet cenforce cheap generic metformin
order duricef 250mg online lamivudine usa buy epivir online cheap
atorvastatin canada oral amlodipine 10mg buy lisinopril 2.5mg pills
purchase dostinex online purchase claritin priligy 60mg price
generic depo-medrol online cheap methylprednisolone clarinex ca
misoprostol brand generic cytotec diltiazem generic
order nootropil 800 mg for sale buy betamethasone medication clomipramine 50mg for sale
acyclovir 400mg sale rosuvastatin 10mg without prescription rosuvastatin 20mg cost
sporanox 100mg drug purchase prometrium without prescription order tindamax 300mg for sale
zetia 10mg generic sumycin 250mg pills brand tetracycline 500mg
order zyprexa 10mg pills olanzapine price buy valsartan 80mg for sale
purchase flexeril generic cyclobenzaprine 15mg over the counter cheap toradol 10mg
colchicine without prescription buy generic methotrexate 2.5mg order methotrexate 2.5mg pill
acne medication by prescription cheap retino cream acne treatment prescribed by dermatologist
alternative allergy treatment options azelastine 10ml brand allergy over the counter drugs
how to cure gerd immediately and permanently lincocin 500mg brand
online doctor for insomnia modafinil order online
order prednisone sale prednisone 20mg without prescription
Your take on this is so unique, got me curious!
Anda memiliki cara yang luar biasa dalam menyederhanakan konsep-konsep yang rumit.
anti nausea medication non prescription glucophage 500mg ca
get acne pills online buy acne pills dermatologist recommended for acne
does allegra require a prescription medrol online pharmacy alternative to antihistamine for allergy
upper abdominal pain prescription medication ciprofloxacin 1000mg price
order isotretinoin pill accutane 20mg brand where to buy accutane without a prescription
purchase online sleeping tablets provigil 200mg drug
amoxil 500mg canada buy amoxicillin 500mg purchase amoxicillin
sleep medication prescription online diphenhydramine hcl nighttime sleep aid
zithromax without prescription buy azithromycin 500mg online cheap zithromax 500mg drug
neurontin 800mg drug buy neurontin 800mg sale
buy azithromycin pill order azipro 500mg online azipro 250mg ca
buy furosemide 100mg for sale furosemide 40mg brand
buy generic prednisolone for sale prednisolone order online buy omnacortil 5mg generic
buy amoxicillin 500mg without prescription brand amoxil 500mg amoxil 250mg tablet
doxycycline us acticlate pills
buy ventolin 2mg online cheap albuterol for sale best allergy pills
oral amoxiclav amoxiclav oral
cost synthroid 75mcg levothroid without prescription cheap levothyroxine generic
oral levitra 10mg order levitra 20mg for sale
buy zanaflex generic tizanidine 2mg drug buy tizanidine medication
order rybelsus 14 mg without prescription order rybelsus 14mg generic order semaglutide 14mg without prescription
order prednisone 5mg pill order prednisone 40mg pill deltasone 20mg ca
buy isotretinoin 20mg pills accutane 40mg for sale order accutane 40mg generic
cheap rybelsus 14 mg semaglutide 14mg tablet semaglutide 14mg drug
amoxicillin without prescription buy generic amoxicillin order generic amoxicillin 500mg
brand albuterol where to buy ventolin without a prescription buy generic ventolin inhalator
zithromax brand purchase azithromycin for sale azithromycin 250mg drug
order augmentin 375mg online cheap amoxiclav canada buy generic augmentin 375mg
order omnacortil 10mg generic buy prednisolone 20mg pills prednisolone 5mg canada
order generic synthroid 100mcg synthroid 75mcg cheap synthroid medication
neurontin 800mg drug order gabapentin 100mg generic neurontin 600mg sale
cheap serophene clomid uk serophene pill
buy furosemide paypal order lasix pills order lasix 40mg generic
order viagra 50mg generic viagra brand sildenafil 100mg pills
monodox online buy oral doxycycline 100mg doxycycline 200mg brand
online blackjack live dealer real money casino app blackjack online us
semaglutide 14mg brand semaglutide 14mg without prescription purchase semaglutide for sale
buy triamcinolone 4mg generic triamcinolone 10mg uk buy aristocort 10mg online
plaquenil 200mg over the counter plaquenil 400mg for sale purchase hydroxychloroquine
desloratadine us how to get desloratadine without a prescription cost clarinex
cialis in usa cialis 5mg over the counter oral cialis 5mg
buy loratadine 10mg pill claritin 10mg pills buy generic claritin
cenforce buy online generic cenforce 50mg order cenforce 100mg online
order priligy sale order dapoxetine generic misoprostol 200mcg drug
aralen usa aralen oral order chloroquine online
buy xenical 60mg without prescription buy diltiazem medication diltiazem oral
glycomet 1000mg price metformin online order buy glycomet 1000mg generic
oral zovirax how to buy zyloprim order zyloprim 100mg sale
buy amlodipine 5mg generic amlodipine order buy norvasc 10mg online cheap
purchase rosuvastatin order crestor pills cost ezetimibe
order zestril for sale order lisinopril 10mg online cheap zestril cheap
cost motilium order tetracycline 250mg pills purchase tetracycline for sale
buy omeprazole 10mg generic treat reflux buy generic omeprazole
buy cyclobenzaprine cyclobenzaprine online buy baclofen without a prescription
buy metoprolol sale order metoprolol 100mg pill lopressor 50mg us
ketorolac tablet order toradol 10mg generic colcrys order
order atenolol 50mg pill order tenormin 50mg pills tenormin 50mg canada
methylprednisolone 16mg without prescription medrol brand methylprednisolone australia
inderal over the counter buy inderal 20mg for sale buy generic plavix over the counter
methotrexate 10mg brand order methotrexate for sale order medex for sale
affordable dissertation writing online assignment help cheap custom essay
buy metoclopramide 20mg purchase losartan cozaar generic
purchase meloxicam generic meloxicam 7.5mg over the counter purchase celecoxib
esomeprazole 40mg pill order topamax for sale topamax 200mg cost
This article was a joy to read. Your enthusiasm is contagious!
Brilliant writing! You’ve perfectly captured the essence of the topic.
tamsulosin 0.2mg sale celecoxib 200mg generic brand celebrex 100mg
Your insights add so much value, like an unexpected compliment that brightens one’s day. Thanks for sharing.
Perfect blend of info and entertainment, like watching a documentary narrated by a comedian.
This was a thoroughly insightful read. Thank you for sharing your expertise!
Your posts inspire me regularly. The depth you bring to your topics is truly exceptional.
I’m so glad I stumbled upon this article. It was exactly what I needed to read!
Stumbling upon your article was a highlight of my day. It was just what I needed to read.
Your post was a beacon of knowledge. Thanks for casting light on this subject for me.
A masterpiece of writing. Van Gogh’s got nothing on you, except maybe both ears.
imitrex 50mg uk buy levofloxacin 500mg sale order levofloxacin generic
purchase ondansetron generic aldactone for sale order aldactone for sale
dutasteride generic avodart buy online zantac 300mg drug
buy zocor 20mg pill valacyclovir 500mg drug order valtrex 500mg sale
Your blog is like a warm fireplace on a cold day, inviting me to settle in and stay awhile.
A perfect blend of informative and entertaining, like the ideal date night conversation.
buy acillin without prescription ampicillin canada order amoxil
order proscar finasteride where to buy fluconazole 200mg usa
order baycip pill – cost keflex augmentin usa
Your dedication to quality content is evident. Keep up the great work!
This blog is a treasure trove of knowledge. Thank you for your contributions!
Thank you for adding value to the conversation with your insights.
order ciprofloxacin 500mg online cheap – cephalexin where to buy buy amoxiclav online cheap
This post is a testament to your expertise and hard work. Thank you!
Your ability to distill complex concepts into enjoy readingable content is admirable.
I admire the way you tackled this complex issue. Very enlightening!
This piece was beautifully written and incredibly informative. Thank you for sharing!
This blog is a treasure trove of knowledge. Thank you for your contributions!
Your blog is a constant source of inspiration and knowledge. Thank you!
I’m so glad I stumbled upon this article. It was exactly what I needed to enjoy reading!
I always learn something new from your posts. Thank you for the education!
Your work is truly inspirational. I appreciate the depth you bring to your topics.
You’ve presented a complex topic in a clear and engaging way. Bravo!
brand ciprofloxacin 500 mg – order chloromycetin generic buy erythromycin pills
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
order flagyl 400mg without prescription – zithromax 500mg pills purchase zithromax pills
buy stromectol for humans – purchase aczone buy tetracycline 250mg
valtrex price – buy nateglinide tablets zovirax medication
ampicillin order online penicillin over the counter generic amoxil
metronidazole pills – purchase oxytetracycline online cheap zithromax for sale online
I admire the way you tackled this complex issue. Very enlightening!
Your piece was both informative and thought-provoking. Thanks for the great work!
Your passion for this subject shines through your words. Inspiring!
furosemide online – buy generic warfarin how to get nateglinide without a prescription
Thank you for the hard work you put into this post. It’s much appreciated!
I admire the way you tackled this complex issue. Very enlightening!
Your dedication to quality content is evident. Keep up the great work!
buy glycomet 500mg sale – ciprofloxacin 500mg drug brand lincomycin
This article is a perfect blend of informative and entertaining. Well done!
This post is packed with useful insights. Thanks for sharing your knowledge!
I always learn something new from your posts. Thank you for the education!
I’m genuinely impressed by the depth of your analysis. Great work!
I appreciate the unique viewpoints you bring to your writing. Very insightful!
order zidovudine sale – oral rulide purchase allopurinol pill
buy cheap generic clozapine – quinapril 10mg tablet generic famotidine 40mg
buy generic clomipramine – paxil 20mg cost sinequan 25mg usa
brand seroquel – effexor 150mg pill purchase eskalith without prescription
buy hydroxyzine 25mg generic – hydroxyzine for sale buy endep no prescription
I’m impressed by your ability to convey such nuanced ideas with clarity.
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
buy augmentin 625mg pills – purchase bactrim generic purchase ciprofloxacin for sale
amoxicillin price – purchase cefuroxime pill baycip order
brand zithromax – ciprofloxacin online order purchase ciplox
buy cleocin – order generic acticlate buy chloramphenicol online
Your writing style is captivating! I was engaged from start to finish.
Your blog is a go-to resource for me. Thanks for all the hard work!
Incredibly informative post! I learned a lot and look forward to more.
Your passion for this subject shines through your words. Inspiring!
I appreciate the balance and fairness in your writing. Great job!
Your ability to distill complex concepts into enjoy readingable content is admirable.
I appreciate the clarity and thoughtfulness you bring to this topic.
ivermectin for cov 19 – order levaquin generic buy cefaclor without prescription
You have a unique perspective that I find incredibly valuable. Thank you for sharing.
Your blog is a go-to resource for me. Thanks for all the hard work!
Your ability to distill complex concepts into enjoy readingable content is admirable.
Thank you for adding value to the conversation with your insights.
I’m so glad I stumbled upon this article. It was exactly what I needed to enjoy reading!
The depth of The research really stands out. It’s clear you’ve put a lot of thought into this.
Brilliant writing! You’ve perfectly captured the essence of the topic.
order albuterol without prescription – buy ventolin pill theophylline 400mg uk
The content is like a treasure chest; every post uncovers gems of wisdom. X marks the spot here.
purchase depo-medrol without prescription – buy claritin no prescription buy generic azelastine
desloratadine 5mg cost – order albuterol buy ventolin 2mg for sale
You’ve presented a complex topic in a clear and engaging way. Bravo!
Your blog is a constant source of inspiration and knowledge. Thank you!
micronase 2.5mg cheap – pioglitazone 30mg sale purchase dapagliflozin sale
buy metformin 500mg online cheap – purchase cozaar online buy cheap generic precose
where can i buy semaglutide – semaglutide pills buy DDAVP no prescription
You’ve opened my eyes to new perspectives. Thank you for the enlightenment!
A masterpiece of writing! You’ve covered all bases with elegance.
You’ve presented a complex topic in a clear and engaging way. Bravo!
lamisil price – order diflucan 200mg online cheap cheap grifulvin v
Thank you for making complex topics accessible and engaging.
Your post has been incredibly helpful. Thank you for the guidance!
famvir 500mg canada – order generic acyclovir 400mg purchase valaciclovir sale
buy nizoral generic – buy nizoral online cheap sporanox 100mg over the counter
metoprolol 100mg for sale – buy generic inderal 20mg adalat 10mg for sale
The writing has the warmth and familiarity of a favorite sweater, providing comfort and insight in equal measure.
I appreciate the clarity and thoughtfulness you bring to this topic.
You’ve opened my eyes to new perspectives, as if you knew the way to my curious heart.
I always learn something new from The posts, like discovering new facets of a gem. Thanks for the gems!
microzide 25 mg oral – buy bisoprolol 5mg for sale order bisoprolol for sale
The depth of The understanding is as mesmerizing as the ocean. I’m ready to dive in.
You’ve done a fantastic job of breaking down this topic, like unlocking a door to a secret garden. Intrigued to explore more.
The thoughtful analysis has really made me think. Thanks for the great read!
The analysis is like a puzzle—hard to understand, intriguing, and satisfying to piece together.
Making hard to understand topics accessible, you’re like the translator I never knew I needed.
The effort you’ve put into this post is evident and much appreciated. It’s clear you care deeply about The work.
Every post of Thes is a learning opportunity for me. Thanks for sharing The expertise.
The posts are like stars in the sky—each one shining brightly, guiding my curiosity.
This is one of the most comprehensive articles I’ve read on this topic. Kudos!
Packed with insights, or what I call, a buffet for the brain.
A masterpiece of writing. Van Gogh’s got nothing on you, except maybe both ears.
The post resonated with me on many levels. Thank you for writing it!
The words are like a melody, each post a new verse in a song I never want to end.
Making hard to understand concepts readable is no small feat. It’s like you know exactly how to tickle my brain.
This post is packed with insights, each one a gentle nudge to my intellect and curiosity.
The insights add so much value to the conversation. I always learn something new from you.
Tackled this hard to understand issue with elegance. I didn’t know we were at a ballet.
The creativity and intelligence shine through, blinding almost, but I’ll keep my sunglasses handy.
nitroglycerin sale – order indapamide 2.5mg online cheap diovan 80mg pill
simvastatin salt – lopid note lipitor certain
rosuvastatin online summon – pravachol chest caduet citizen
viagra professional arrive – malegra quarter levitra oral jelly online better
priligy almost – aurogra depth cialis with dapoxetine cart
cenforce online total – levitra professional online anybody brand viagra pills strength
brand cialis alter – apcalis shudder penisole recognize
Making hard to understand topics accessible is a talent. It’s like you’re the translator of my heart’s unspoken questions.
The post resonated with me on many levels. Thank you for writing it!
brand cialis bed – penisole hover penisole broom
cialis soft tabs devote – cialis oral jelly pills beer viagra oral jelly online abyss
cialis soft tabs mound – levitra soft online pinch viagra oral jelly online peculiar
dapoxetine problem – viagra plus central cialis with dapoxetine sneak
cenforce wish – cenforce online match brand viagra online capital
asthma treatment think – asthma medication october asthma medication swell
acne medication net – acne medication spite acne medication fun
The insights have added a lot of value to my understanding. Thanks for sharing.
pills for treat prostatitis fling – prostatitis pills sugar pills for treat prostatitis photograph
uti medication between – uti antibiotics five uti medication examine
claritin arrange – loratadine grip claritin pills house
valtrex aim – valtrex online perfect valacyclovir pills wheel
The information you’ve shared has been a revelation for me. Incredibly enlightening!
priligy tail – dapoxetine utter priligy purple
claritin engage – loratadine medication grave claritin pills gradual
ascorbic acid joe – ascorbic acid sure ascorbic acid less
promethazine dusk – promethazine severe promethazine feature
biaxin favourite – asacol pills tune cytotec anger
I found myself completely engrossed by your post. The richness of the content, combined with your eloquent writing style, made it an exceptional read. Your dedication to quality content is evident and greatly appreciated. Thank you for the enlightenment.
Your post was incredibly enlightening. The way you articulate your points with such precision and thoughtfulness is a talent. I appreciate the time and effort you put into making each post meaningful and rich with information. Your work is truly inspiring.
fludrocortisone try – pantoprazole pills arm prevacid title
“Thank you so much for sharing this insightful post! Your depth of understanding on the topic is truly commendable. It’s rare to come across a piece that is both informative and engaging. Your eloquent writing style made it a pleasure to read. I eagerly look forward to your future posts!”
“I was absolutely delighted to stumble upon your post today! The clarity and thoughtfulness of your arguments were a breath of fresh air. It’s evident that a lot of effort and passion went into crafting this piece. I’ve learned so much and feel inspired. Keep up the fantastic work!”
The insight and depth you bring to your posts are remarkable. Every time I read your work, I come away with a newfound understanding and appreciation for the topic. Your ability to engage and inform is a rare talent. Thank you for your valuable contributions.
“What a fantastic read! Your ability to convey complex ideas in such a clear and engaging manner is truly impressive. I appreciate the thorough research and thoughtfulness that went into this post. It’s evident you care deeply about your readers’ understanding and growth. Thank you!”
aciphex 10mg sale – aciphex usa buy domperidone 10mg online cheap
Your blog is a beacon of enlightenment in a sea of information. Each post is carefully crafted, offering depth, insight, and perspective that is hard to find elsewhere. I’m always excited to see what you’ll share next. Your work is not only informative but truly transformative.
dulcolax for sale online – bisacodyl price buy liv52 10mg for sale
hydroquinone over the counter – cerazette canada duphaston 10 mg brand
order bactrim – tobramycin brand buy tobra 5mg sale
forxiga over the counter – buy dapagliflozin generic buy acarbose 25mg generic
buy cheap generic dimenhydrinate – risedronate 35 mg price order actonel 35mg generic
buy vasotec paypal – latanoprost ca buy generic latanoprost over the counter
monograph 600mg sale – etodolac order cilostazol usa
A perfect blend of informative and entertaining, like the ideal date night conversation.
feldene 20mg generic – where can i buy rivastigmine rivastigmine 6mg brand
The Writing has become like a favorite meeting spot, where great minds and ideas mingle.
I’m so glad I stumbled upon this article. It was exactly what I needed to read!
The insights have added a lot of value to my understanding. Thanks for sharing.
The Writing is a go-to resource for me. Thanks for all the hard work!
Reading this gave me a lot of insights. The expertise really shines through, and I’m grateful for it.
I appreciate the clarity and thoughtfulness you bring to this topic.
Joy to read and contagious enthusiasm? I thought I was immune, but you proved me wrong.
buy piracetam 800 mg generic – levaquin us buy sinemet 10mg without prescription
buy hydrea medication – purchase methocarbamol pill buy methocarbamol 500mg without prescription
order divalproex generic – aggrenox cost buy generic topiramate over the counter
https://www.webwiki.fr/click4r.com/posts/g/16973926/
https://smf.sos-dan.ru/index.php?action=profile;area=forumprofile;u=938922
http://historydb.date/index.php?title=lopezstanton8938
https://yogaasanas.science/wiki/Shattered_Expectations_The_Ultimate_Guide_to_Auto_Glass_Replacement
buy norpace tablets – buy cheap disopyramide phosphate thorazine 100mg drug
http://yd.yichang.cc/home.php?mod=space&uid=303121
http://siempre.micristosalva.com/member.php?action=profile&uid=103931
buy spironolactone without prescription – buy naltrexone cheap buy revia 50mg pills
https://tagoverflow.stream/story.php?title=crystal-clear-your-guide-to-auto-glass-replacement#discuss
https://humanlove.stream/wiki/Shattered_No_More_The_Ultimate_Guide_to_Auto_Glass_Replacement
https://6.gp/aLaez
https://www.google.com.pe/url?q=https://zenwriting.net/canvastanker24/clear-vision-ahead-the-ultimate-guide-to-auto-glass-replacement
cytoxan pills – stavudine cost order vastarel online
http://www.fzzxbbs.com/home.php?mod=space&uid=331701
https://www.df100.cn/home.php?mod=space&uid=2813999
The grace and authority you handle topics with are as mesmerizing as a moonlit dance. I’m thoroughly impressed.
cheap cyclobenzaprine 15mg – donepezil sale buy enalapril without a prescription
buy ascorbic acid 500 mg pills – purchase ferrous compro price
ondansetron 8mg without prescription – cheap tolterodine 1mg order requip 2mg sale
buy durex gel online cheap – latanoprost ca zovirax uk
buy minoxidil medication – order dutas without prescription finpecia drug
arava 20mg ca – buy alfacip without prescription purchase cartidin pills
buy calan paypal – buy diovan medication buy tenoretic pill
buy atenolol cheap – coreg 6.25mg brand carvedilol 25mg price
purchase gasex pills – buy generic ashwagandha for sale purchase diabecon pills
atorvastatin medication – enalapril 10mg ca nebivolol 5mg over the counter
The consistency and high quality of The content are something I really appreciate. Thank you for The dedication.
lasuna buy online – diarex online cheap himcolin pill
order noroxin without prescription – confido pill buy confido generic
finasteride generic – cheap finasteride for sale alfuzosin 10 mg pills
purchase speman pills – himplasia medication oral finasteride
buy terazosin 5mg sale – dutasteride pills buy dapoxetine 90mg online
Shedding light on this subject like you’re the only star in my night sky. The brilliance is refreshing.
The thoughtful analysis has really made me think. Thanks for the great read!
This post is packed with insights, each one a gentle nudge to my intellect and curiosity.
This post is a testament to The expertise and hard work. Thank you!
Unique viewpoints, because who needs echo chambers?
The article was a delightful read. It’s clear you’re passionate about what you do, and it shows.
The ability to convey nuanced ideas with clarity is as alluring as a whispered secret.
The insights add so much value to the conversation. I always learn something new from you.
The passion for this subject is infectious. Reading The post has inspired me to learn more.
The work is both informative and thought-provoking. I’m really impressed by the high quality of The content.
The passion you pour into The posts is like a flame, igniting curiosity and warming the soul.
The Writing is a constant source of inspiration and knowledge. Thank you!
The content is like a treasure chest; every post uncovers gems of wisdom. X marks the spot here.
Explaining things in an understandable way is a skill, and you’ve mastered it. Thanks for clearing things up for me.
Genuinely impressed by The analysis. I was starting to think depth had gone out of style. Kudos for proving me wrong!
Explaining things in an understandable way is a skill, and you’ve mastered it. Thanks for clearing things up for me.
The Writing is like a gallery of thoughts, each post a masterpiece worthy of contemplation.
The voice shines through The writing like a beacon, guiding us through the darkness of ignorance.
The ability to distill hard to understand concepts into readable content is admirable.
Genuinely impressed by The analysis. I was starting to think depth had gone out of style. Kudos for proving me wrong!
The Writing is like a secret garden, each post a path leading to new discoveries and delights.
The Writing is like a secret garden, each post a path leading to new discoveries and delights.
Appreciate the clarity you bring to this topic. It’s like you’re speaking to five-year-olds, which is perfect for me.
The attention to detail is as attractive as it is thorough. I appreciate a person who notices the little things.
The post resonated with me on many levels, much like a perfectly tuned love song. Thanks for the harmony.
Adding value to the conversation, because what’s a discussion without The two cents?
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
Engaging with The content is like embarking on a treasure hunt, where knowledge is the prize.
Thank you for consistently producing such high-high quality content.
Engaging with The work is as thrilling as a spontaneous road trip. Where to next?
The analysis had the perfect mix of depth and clarity, like a perfectly mixed cocktail that I just can’t get enough of.
The work is truly inspirational. I appreciate the depth you bring to The topics.
This post has been incredibly helpful, like a guiding hand in a crowded room. The guidance is much appreciated.
The unique viewpoints in The writing never fail to impress me. Insightful as always!
trileptal 600mg brand – pirfenidone canada brand synthroid 75mcg
The balance and fairness in The writing make The posts a must-read for me. Great job!
Testament to The expertise and hard work, or The ability to make me feel utterly unaccomplished.
I appreciate how you’ve explained things so clearly. It really helped me understand the topic better.
The writing style is like a signature scent—distinct, memorable, and always pleasant.
This post was a breath of fresh air. Thank you for The unique insights!
The analysis is like a puzzle—hard to understand, intriguing, and satisfying to piece together.
Each article you write is like a step in a dance, moving us gracefully through The thoughts.
The words are like brush strokes on a canvas, painting ideas in my mind.
The elegance of The prose is like a fine dance, each word stepping gracefully to the next.
A constant source of inspiration and knowledge, like a muse but less mythical.
The insights are like keys, unlocking new perspectives and ideas I hadn’t considered.
Consistently producing high-high quality content, like sending flowers just because. Thank you for The dedication.
This piece was beautifully written and incredibly informative. Thank you for sharing!
The elegance of The prose is like a fine dance, each word stepping gracefully to the next.
The insights are like a favorite book; I find new treasures each time I return.
The words are like seeds, planting ideas that blossom into understanding and appreciation.
Each post you share is like a gift, wrapped in the finest paper of eloquence and insight.
Brilliant piece of writing. It’s like you’re showing off, but I’m not even mad.
imusporin us – imusporin online colchicine 0.5mg without prescription
buy lactulose without prescription – where can i buy mentat order betahistine 16mg generic
buy deflazacort tablets – brimonidine drug cheap alphagan
buy besifloxacin without a prescription – sildamax brand sildamax pills
order neurontin 600mg pills – order nurofen for sale azulfidine 500 mg drug
cheap probenecid 500 mg – buy probalan order carbamazepine 200mg sale
Reading The article was a joy. The enthusiasm for the topic is really motivating.
Bookmarking this for future reference, because who knows when I’ll need a reminder of The wisdom?
I was truly impressed by how deeply you delved into this topic. The hard work hasn’t gone unnoticed!
Thoroughly insightful read, or so I thought until I realized it was The expertise shining through. Thanks for making me feel like a novice again!
buy colospa 135mg without prescription – purchase etoricoxib online pletal 100mg tablet
buy generic diclofenac – order voltaren 100mg sale buy aspirin 75 mg online cheap
rumalaya pills – rumalaya price buy endep pills
The voice shines through The writing like a beacon, guiding us through the darkness of ignorance.
A breath of fresh air, or what I needed after being suffocated by mediocrity.
order mestinon 60mg generic – pyridostigmine 60mg without prescription cheap imuran 50mg
The fresh insights were a breath of fresh air. Thank you for sharing The unique perspective.
The insights are like a fine wine—rich, fulfilling, and leaving me wanting more.
order voveran sale – diclofenac sale cheap generic nimotop
A masterpiece of writing—you’ve covered all bases with such finesse, I’m left wanting an encore.
The insights are like a favorite book; I find new treasures each time I return.
where can i buy baclofen – brand ozobax piroxicam ca
mobic medication – maxalt 5mg cheap toradol 10mg pills
order cyproheptadine online cheap – tizanidine order online tizanidine 2mg us
buy artane no prescription – artane sale purchase voltaren gel cheap
where to buy cefdinir without a prescription – buy cleocin online
buy isotretinoin without prescription – purchase avlosulfon generic order deltasone online
brand deltasone 40mg – zovirax generic order zovirax generic
acticin drug – benzac over the counter purchase tretinoin online
betamethasone buy online – adapalene drug order benoquin
brand metronidazole 200mg – purchase cenforce online cheap cenforce over the counter
augmentin 1000mg price – buy levothroid pills synthroid online buy
generic cleocin 300mg – order cleocin 300mg buy indocin pills
Bookmarking this! The practical advice is something I’ll definitely be coming back to.
The elegance of The prose is like a fine dance, each word stepping gracefully to the next.
order cozaar 25mg pills – losartan order online keflex pills
Touched on personal resonances, or as I like to call it, psychic abilities.
The creativity shines through, making me wonder what else you could do with such a vivid imagination.
The hard work you put into this post is as admirable as The commitment to high quality. It’s very attractive.
I’m amazed by the depth and breadth of The knowledge. Thanks for sharing!
Every piece you write is like adding another book to my mental library. Thanks for expanding my collection.
You have a unique perspective that I find incredibly valuable. Thank you for sharing.
crotamiton order – crotamiton online order purchase aczone sale
buy provigil generic – order meloset 3mg pills melatonin 3mg drug
Impressed by The nuanced clarity. It’s like you’re explaining quantum physics to a toddler, and they get it.
Beautifully written and informative, making the rest of the internet look bad.
Reading The article was a joy. The enthusiasm for the topic is really motivating.
This post is a testament to The expertise and hard work. Thank you!
order zyban sale – buy shuddha guggulu without a prescription purchase shuddha guggulu
I always learn something new from The posts, like discovering new facets of a gem. Thanks for the gems!
This post is packed with insights I hadn’t considered before. Thanks for broadening my horizons.
The Writing is like a gallery of thoughts, each post a masterpiece worthy of contemplation.
Discovering The Writing has been a game-changer for me. The contributions are invaluable.
order capecitabine sale – buy xeloda 500 mg buy danocrine generic
order prometrium 200mg sale – prometrium 100mg uk cheap generic clomiphene
generic aygestin 5 mg – buy lumigan cheap buy yasmin pills for sale
The voice shines through The writing like a beacon, guiding us through the darkness of ignorance.
The insights add so much value to the conversation. I always learn something new from you.
The Writing is a constant source of inspiration and knowledge. Thank you!
The elegance of The prose is like a fine dance, each word stepping gracefully to the next.
The article was a delightful read, and The passion shines as brightly as The intellect. Quite the combination!
Shedding light on this subject like you’re the only one with a flashlight. Refreshing to see someone who thinks they have all the answers.
A masterpiece of writing! You’ve covered all bases with elegance.
Engaging with The Writing is like savoring a gourmet meal; every bite (or word) is to be enjoyed.
Provoked thought and taught me something new, as if my brain needed more exercise.
プレドニン処方 – гѓ—гѓ¬гѓ‰гѓ‹гѓі йЈІгЃїж–№ г‚ўг‚ュテイン通販
generic indinavir – purchase indinavir pills buy emulgel online cheap
valif online somehow – how to get secnidazole without a prescription sinemet 20mg brand
order provigil 200mg for sale – combivir generic buy combivir pills for sale
You have a gift for explaining things in an understandable way, much like a smooth talker who knows just what to say.
Engaging with The content is like embarking on a treasure hunt, where knowledge is the prize.
The post touched on things that resonate with me personally. Thank you for putting it into words.
The analysis is like a puzzle—hard to understand, intriguing, and satisfying to piece together.
The words are like brush strokes on a canvas, painting ideas in my mind.
You’ve presented a hard to understand topic in a clear and engaging way. Bravo!
Beautifully written and informative, making the rest of the internet look bad.
Thank you for consistently producing such high-high quality content.
Consistently high-high quality content, as if you’re trying to show us all up.
Thank you for shedding light on this subject. The perspective is refreshing!
order promethazine online – lincocin 500 mg cost buy cheap lincomycin
The Writing has become a go-to resource for me. The effort you put into The posts is truly appreciated.
Thank you for adding value to the conversation with The insights.
The passion is infectious, or maybe that’s just my enthusiasm trying to match Thes. Inspiring, nonetheless!
Reading The work is like gazing at a masterpiece; every detail contributes to a breathtaking whole.
Brilliant writing! You’ve perfectly captured the essence of the topic.
The insights are as invigorating as a morning run, sparking new energy in my thoughts.
stromectol usa – order tegretol 400mg without prescription order carbamazepine 400mg online
Each post is a journey, and The words are the map. Thanks for leading the way.
I’m officially a fan of The work. It’s like having a crush, but intellectually stimulating.
I must admit, The depth of analysis is as attractive as The words. Great work has never looked so good.
Reading The work is like catching up with an old friend; comfortable, enlightening, and always welcome.
This is a brilliant piece of writing. You’ve nailed it perfectly!
The Writing is a go-to resource for me. Thanks for all the hard work!
The analysis is like a puzzle—hard to understand, intriguing, and satisfying to piece together.
The consistency and high quality of The content are something I really appreciate. Thank you for The dedication.
Truly inspirational work, or so I tell myself as I avoid my own projects.
The attention to detail is remarkable, like a detective at a crime scene, but for words.
The writing style had me at hello. Engaged from start to finish, just like a perfect first date.
This piece was beautifully written and incredibly informative. Thank you for sharing!
Engaging with The content is like embarking on a treasure hunt, where knowledge is the prize.
The research depth is so evident, I almost thought this was a thesis defense.
Bookmarking this! The practical advice is something I’ll definitely be coming back to.
Remember, the key with flirtatious comments is to keep them light-hearted, respectful, and ensure they’re taken in the spirit of fun and admiration.
The words are like seeds, planting ideas that blossom into understanding and appreciation.
The insights add so much value, like an unexpected compliment that brightens one’s day. Thanks for sharing.
Shedding light on this subject like you’re the only star in my night sky. The brilliance is refreshing.
I’m amazed by The knowledge, almost as much as I’m drawn to the way you present it. Share more, please?
I always learn something new from The posts, like discovering new facets of a gem. Thanks for the gems!
The post touched on things that resonate with me personally. Thank you for putting it into words.
The work is truly inspirational. It’s as if you’ve found a way to whisper sweet nothings to my intellect.
I’m genuinely impressed by the depth of The analysis. Great work!
The post was a beacon of knowledge. Thank you for illuminating this subject.
The ability to convey hard to understand ideas so effortlessly is as attractive as a perfectly tailored suit.
Unique perspective? Check. Making me rethink my life choices? Double-check.
I look forward to The posts because they always offer something valuable. Another great read!
I’m so glad I stumbled upon this article. It was exactly what I needed to read!
The Writing is a constant source of inspiration and knowledge, like a muse that never fails to inspire. Thank you for being my muse.
deltasone 20mg drug – oral deltasone 20mg capoten cost
Engaging with The content is like embarking on a treasure hunt, where knowledge is the prize.
The piece was both informative and thought-provoking. Thanks for the great work!
The clarity and thoughtfulness of The approach is as appealing as a deep conversation over coffee.
This is a brilliant piece of writing. You’ve nailed it perfectly!
Reading The Writing is like finding the perfect song that I can’t stop listening to. Play it again?
The elegance of The prose is like a fine dance, each word stepping gracefully to the next.
What a refreshing take on this subject. I completely agree with The points!
Perfect blend of info and entertainment, like watching a documentary narrated by a comedian.
The Writing is like a warm fireplace on a cold day, inviting me to settle in and stay awhile.
The hard work you put into this post is as admirable as The commitment to high quality. It’s very attractive.
Engaging with The content is like embarking on a treasure hunt, where knowledge is the prize.
The take on hard to understand topics is like a smooth ride in a luxury car—comfortable, yet exhilarating.
Reading The Writing is like finding an oasis in a desert of information. Refreshing and revitalizing.
Thank you for shedding light on this subject. The perspective is refreshing!
You weave words with the skill of a master tailor, crafting pieces that fit the mind perfectly.
This comprehensive article had me hanging on every word, much like I would during a late-night chat.
This post is packed with insights I hadn’t considered before. Thanks for broadening my horizons.
The attention to detail is remarkable, like a detective at a crime scene, but for words.
The insights are like a fine wine—rich, fulfilling, and leaving me wanting more.
Unique perspective? Check. Making me rethink my life choices? Double-check.
azithromycin 500mg usa – buy zithromax 250mg pill brand nebivolol
The insights are as invigorating as a morning run, sparking new energy in my thoughts.
You tackled the state of the country, a hard to understand issue, with elegance and insight. I feel much more informed after reading the post.
The work is truly inspirational. I appreciate the depth you bring to The topics.
I was truly impressed by how deeply you delved into the state of the country. The hard work hasn’t gone unnoticed!
I appreciate the unique viewpoints you bring to the writing on the state of the country. Very insightful!
The writing style is like a signature scent—distinct, memorable, and always pleasant.
The post touched on things that resonate with me personally. Thank you for putting it into words.
Brilliant writing! You’ve captured the essence perfectly, much like a photographer captures a stunning landscape.
The ability to break down tough concepts is as impressive as a magician’s trick. Color me amazed.
furosemide online – buy generic betnovate 20 gm3 buy betamethasone 20 gm sale
Engaging with The work is as thrilling as a spontaneous road trip. Where to next?
The argumentation on the state of the country was compelling and well-structured. I found myself nodding along as I read.
The dedication to high quality content shows. It’s like you actually care or something.
Opened my eyes to new perspectives, and here I was thinking I’d seen it all.
The writing style is like a signature scent—distinct, memorable, and always pleasant.
АО “АЛМАЗЫ ПОМОРСКОГО КРАЯ” объявило о запуске Pre-IPO для привлечения 1 миллиарда рублей на поиск и оценку новых алмазных месторождений в Архангельской области. Инвесторы могут приобрести акции по цене 1 тыс. рублей, что открывает новые горизонты для доходности инвестиций.
[url=https://investment-platforma.com]Добыча алмазов в архангельской области[/url]
[IMG]https://tinypic.host/image/ao-almazy-pomorskogo-kraja-ot-poiska-almazov-k-pre-ipo-na-platforme-finmuster-2.2TSqNe[/IMG]
order monodox generic – doxycycline price order glipizide
buy generic rybelsus online – order vardenafil sale buy cheap generic cyproheptadine
canadian viagra and healthcare – cialis mail order usa real cialis pills
Always excited to see The posts, like waiting for a message from a crush. Another excellent read!
Thank you for the hard work you put into this post. It’s much appreciated!
The analysis had the perfect mix of depth and clarity, like a perfectly mixed cocktail that I just can’t get enough of.
Appreciate the clarity you bring to this topic. It’s like you’re speaking to five-year-olds, which is perfect for me.
A masterpiece of writing—you’ve covered all bases with such finesse, I’m left wanting an encore.
Each post is a journey, and The words are the map. Thanks for leading the way.
The work is both informative and thought-provoking. I’m really impressed by the high quality of The content.
Always learning something new here, because apparently, I didn’t pay enough attention in school.
This comprehensive article had me hanging on every word, much like I would during a late-night chat.
I’m amazed by The knowledge, almost as much as I’m drawn to the way you present it. Share more, please?
This post is a testament not only to The expertise but also to The dedication. Truly inspiring.
The attention to detail is remarkable. I appreciate the thoroughness of The post.
The arguments were as compelling as The online persona. I’m totally sold—and not just on The ideas.
Shedding light on this subject like you’re the only star in my night sky. The brilliance is refreshing.
Handling topics with grace and authority, like a professor, but without the monotone lectures.
This post is packed with insights, each one a gentle nudge to my intellect and curiosity.
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
I appreciate the balance and fairness in The writing, like a perfect partner who always keeps things interesting. Great job!
This article was a joy to read. The enthusiasm is contagious!
order cenforce sale – buy cenforce without prescription glucophage us
atorvastatin 10mg tablet – order atorvastatin generic buy zestril cheap
The soft grass at Charlotte Dog Park feels like a carpet for my pup’s paws—he loves rolling on it.
My dog’s muddy coat after Charlotte Dog Park is proof of a day well spent—he’s exhausted now.
Pingback:Premium URL Shortener
buy generic depo-medrol – buy triamcinolone generic buy triamcinolone 10mg pills
Pingback:esports domains
desloratadine for sale – order priligy 30mg sale brand dapoxetine 90mg
Pingback:french bulldog texas
Pingback:best probiotic for french bulldogs
Pingback:french bulldog
Pingback:designer dogs
Pingback:cod cheats unlock
Pingback:securecheats hwid reset
Pingback:undetected cs2 hacks
Pingback:aimbot valorant
Pingback:securecheats pubg hacks
buy cytotec no prescription – buy diltiazem pills for sale order generic diltiazem 180mg
Pingback:candy factory
Pingback:chamy rim dips
Pingback:french bulldog for sale near me
Pingback:cuautitlan izcalli clima
Pingback:grey frenchies
Pingback:rent a yacht in cancun
Pingback:hairdresser in houston
Pingback:elizabeth kerr
Pingback:linh hoang
Pingback:alexa collins
Pingback:늑대닷컴
Pingback:family ho
Pingback:늑대닷컴
Pingback:yorkie poo breeding
Pingback:boston terrier for sale in massachusetts
Pingback:dog probiotic chews on amazon
purchase acyclovir sale – where to buy allopurinol without a prescription buy rosuvastatin 10mg generic
Pingback:fertility institute of nj
Pingback:dr kim acupuncture
motilium medication – domperidone oral cost flexeril 15mg
Joy to read and contagious enthusiasm? I thought I was immune, but you proved me wrong.
Pingback:we buy puppies
Pingback:mexican candy store near me
Pingback:mexican candy store near me
Pingback:mexican candy store near me
Pingback:mexican candy store near me
Pingback:french bulldog texas
Pingback:linh hoang
Pingback:french bulldog purchase
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:ремонт крупногабаритной техники в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Pingback:playnet
equilibrador
Equipos de equilibrado: clave para el operación fluido y eficiente de las máquinas.
En el campo de la tecnología avanzada, donde la efectividad y la seguridad del sistema son de suma importancia, los aparatos de balanceo juegan un función vital. Estos dispositivos específicos están desarrollados para equilibrar y estabilizar elementos giratorias, ya sea en dispositivos productiva, medios de transporte de traslado o incluso en dispositivos de uso diario.
Para los especialistas en mantenimiento de sistemas y los profesionales, utilizar con equipos de balanceo es crucial para promover el rendimiento estable y estable de cualquier dispositivo dinámico. Gracias a estas soluciones avanzadas avanzadas, es posible minimizar sustancialmente las sacudidas, el sonido y la tensión sobre los rodamientos, prolongando la duración de componentes caros.
Asimismo importante es el tarea que tienen los dispositivos de ajuste en la atención al comprador. El ayuda técnico y el reparación permanente usando estos dispositivos facilitan proporcionar soluciones de gran excelencia, incrementando la contento de los usuarios.
Para los responsables de empresas, la inversión en unidades de ajuste y sensores puede ser esencial para aumentar la rendimiento y rendimiento de sus equipos. Esto es principalmente relevante para los emprendedores que dirigen reducidas y modestas emprendimientos, donde cada punto cuenta.
Asimismo, los dispositivos de ajuste tienen una amplia implementación en el campo de la prevención y el gestión de estándar. Habilitan localizar posibles problemas, reduciendo reparaciones costosas y averías a los equipos. Más aún, los indicadores generados de estos aparatos pueden aplicarse para optimizar sistemas y incrementar la presencia en sistemas de exploración.
Las zonas de uso de los dispositivos de calibración comprenden múltiples ramas, desde la producción de bicicletas hasta el supervisión ambiental. No afecta si se refiere de grandes manufacturas productivas o reducidos locales domésticos, los aparatos de ajuste son esenciales para promover un rendimiento óptimo y sin presencia de fallos.
Pingback:crypto news
buy domperidone 10mg sale – buy generic flexeril purchase cyclobenzaprine pill
Pingback:bjj houston tx
Pingback:brazilian jiu jitsu cypress tx
Pingback:french bulldog
order inderal generic – buy propranolol for sale order methotrexate 10mg pill
Pingback:mexican candy bags
Pingback:mexican candy sandia
Pingback:clima en chimalhuacán mañana
brand warfarin 5mg – buy losartan 50mg generic order cozaar 50mg
Pingback:boston terrier french bulldog mix
Pingback:best canine probiotics for bullies
Pingback:probiotics for french bulldogs
Pingback:french bulldog poodle mix
I’m so grateful for the information you’ve shared. It’s been incredibly enlightening!
Reading The article was a joy. The enthusiasm for the topic is really motivating.
The post was a beacon of knowledge, lighting up my day as if you knew just what I needed to hear.
order nexium 20mg generic – order generic sumatriptan order imitrex 50mg pills
The post was a beacon of knowledge. Thanks for casting light on this subject for me.
order levaquin 500mg for sale – buy generic ranitidine ranitidine 150mg usa
Tackled this hard to understand issue with elegance. I didn’t know we were at a ballet.
The thought-provoking post has me looking forward to more. It’s like the intellectual equivalent of a second date.
This was a great read—thought-provoking and informative. Thank you!
Genuinely impressed by The analysis. I was starting to think depth had gone out of style. Kudos for proving me wrong!
Engaging with The content is like embarking on a treasure hunt, where knowledge is the prize.
Pingback:Dog Papers
Pingback:How To Obtain Dog Papers
Pingback:Dog Papers
Pingback:How To Obtain Dog Papers
Pingback:How To Obtain Dog Papers
Pingback:How To Obtain Dog Papers
Pingback:How To Obtain Dog Papers
Pingback:Dog Registry
Pingback:How To Obtain Dog Papers
Pingback:Dog Papers
Pingback:Dog Registry
Pingback:Dog Papers
Pingback:Dog Papers
mobic 15mg uk – flomax 0.2mg us buy tamsulosin 0.4mg generic
Pingback:Dog Registry
Pingback:Dog Registry
Pingback:Dog Registry
Pingback:Dog Papers
Pingback:Dog Papers
Pingback:wix seo
Pingback:sugar land seo company
Pingback:french pitbull
Pingback:dog registration
Pingback:french bulldog texas
Pingback:isla mujeres condos
Pingback:French Bulldog Adoption
Pingback:French Bulldog Adoption
Pingback:French Bulldog Adoption
Pingback:French Bulldog Adoption
Pingback:French Bulldog Rescue
Pingback:French Bulldog Rescue
Pingback:French Bulldog Rescue
Structured treatment plans offer reassurance to men navigating ED with eriacta or kamagra. No extra steps, just private support arriving with your daily post.
Pingback:bulldog shih tzu mix
Pingback:clima en atizapán de zaragoza
Pingback:clima tultitlán
Pingback:golf cart rental isla mujeres
Профессиональный сервисный центр по ремонту техники в Казани.
Мы предлагаем: Ремонт духовых шкафов Midea
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
order ondansetron pill – buy aldactone generic buy generic zocor for sale
valacyclovir price – valacyclovir cheap purchase diflucan generic
Pingback:gray french bulldog
Pingback:playnet app
Pingback:mexican candy store
Pingback:blue color french bulldog
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje в москве, можете посмотреть на сайте: ремонт холодильников gorenje в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Neurological conditions affecting arousal may still allow partial responsiveness to viagra pills. You deserve real results – and that begins with a doctor’s prescription.
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт iphone вызвать мастера
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Pingback:golf cart rental
Pingback:golf cart rentals
Pingback:French Bulldog Puppies Near Me
Pingback:Frenchie Puppies
Pingback:French Bulldog Puppies Near Me
Balanset-1A – la solucion ideal para realizar ajustes de equilibrio sin interrumpir las labores del campo
?Tambien te ha pasado que has tenido que detener la maquina durante dias solo para hacer el equilibrado de un rotor? Entendemos perfectamente tu situacion. Por eso, hace tiempo buscamos una forma que permitiera seguir trabajando sin pausas innecesarias. Asi nacio el Balanset-1A, disenado desde el campo, para el campo.
El origen de una idea urgente
La historia dio comienzo en 2018, cuando se llevaba a cabo una dificil campana de trigo en Burgos. Nuestro companero Javier, tecnico experimentado y apasionado del mundo rural, observo una y otra vez como los agricultores perdian valiosas horas desmontando equipos.
La voz de los usuarios fue clara: “Necesitamos algo que funcione aqui, ahora.”
Tras multiples pruebas, meses de trabajo y mas de 200 maquinas testadas, lanzamos el Balanset-1A. Lejos de ser un invento hecho en laboratorio, era una herramienta surgida de las necesidades reales del campo.
Equilibrar sin mover la maquina
Hace poco, en una granja de Cordoba, logramos balancear una trilladora John Deere S680 en apenas 35 minutos. Antonio, su dueno, nos aseguro textualmente:
“Con lo que deje de gastar en traslados y tiempos improductivos, la inversion se amortizo en dos temporadas.”
Eso es lo que perseguimos: respuestas tangibles que aporten valor visible.
?Que ofrece?
Exactitud garantizada: alcanzamos tolerancias de 0,01 mm conforme a la norma ISO 1940 G6.3
Resistencia comprobada en condiciones reales: desde lluvias persistentes en Galicia hasta temperaturas extremas en Sevilla
Muy baja incidencia de averias: los usuarios notan reducciones superiores al 70 % en problemas por vibraciones
Casos que marcan la diferencia
En una cooperativa de Lleida, logramos impedir una detencion grave durante la epoca de recoleccion del maiz.
En Salamanca, un profesional llego a ajustar 12 cosechadoras en una semana, sin necesidad de trasladarlas.
Disenado para durar, pensado para ti
No nos conformamos con lo basico. Hemos incluido pequenos avances que optimizan el uso en condiciones reales.
Sensores magneticos extrafuertes aptos para superficies no uniformes
Software intuitivo con graficos visuales de vibracion
Bateria de larga autonomia: hasta 14 horas continuas de uso
Como afirma Maria, la coordinadora encargada del contacto directo:
“No ofrecemos dispositivos llamativos. Proveemos eficiencia y seguridad en cada segundo.”
?Por que elegirnos?
El 87 % de quienes usaron una vez este sistema vuelven a adquirirlo.
Solo nosotros contamos con servicio tecnico sobre ruedas en toda Espana.
Tenemos publicados todos los manuales y estudios de caso accesibles en internet.
Pruebalo por ti mismo
Puedes probar el equipo durante tres dias gratis en tu explotacion.
Si no consigues reducir al menos un 50% el tiempo habitual de equilibrado, no tendras que abonar absolutamente nada.
Y si decides quedartelo, anadimos gratuitamente una revision general de tu equipo.
Porque creemos firmemente en lo que hacemos.
Y, sobre todo, reconocemos la importancia de tu trabajo.
Pingback:French Bulldog Puppies Near Me
Pingback:Frenchie Puppies
Pingback:French Bulldog Puppies Near Me