What to Know About the J&J COVID Vaccine Pause
[ad_1]
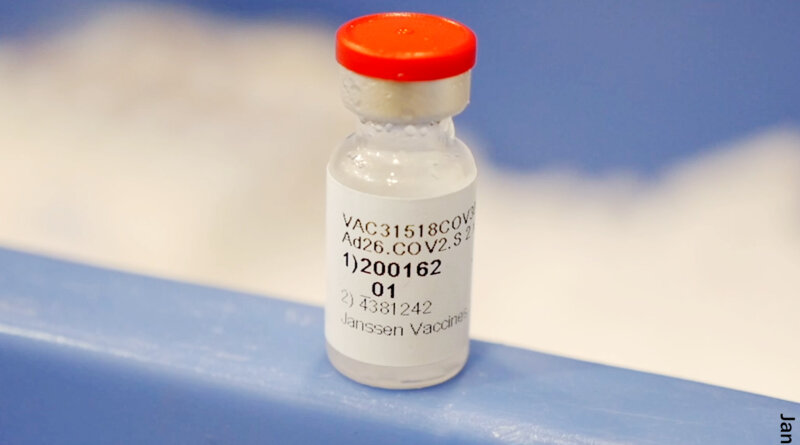
April 13, 2021 — After receiving reports of a rare blood clot in people receiving the Johnson & Johnson COVID-19 vaccine, the FDA and the CDC have recommended a pause in the use of the vaccine, pending further investigation.
Here, what you need to know:
Why was the pause suggested?
After reviewing data, the agencies found six reports of people who experienced rare blood clots in combination with low platelets, which are the smallest blood cells and involved in clotting. The six reports were found out of more than 6.8 million doses given. The FDA and CDC initiated the pause ”out of an abundance of caution.”
What else is known?
According to a joint statement issued by the CDC and FDA, all six cases were in women between ages 18-48. Symptoms occurred 6-13 days after vaccination. The type of clot is known medically as a cerebral venous sinus thrombosis (CVST) and was seen in combination with low levels of blood platelets. Go here to learn more about these types of blood clots.
Of the six cases, one woman died; another was in critical condition.
Treatment of this type of clot is different from that for other types, the CDC and FDA say. Typically, an anticoagulant drug, heparin, is used. But for this type of clot, alternative treatment may be needed.
Why does it happen?
“We don’t have a definitive answer at this time, but it appears to involve an immune response related to the J&J vaccine that adversely affects the function of the platelets, which in turn prevents the normal clotting process from occurring,” says Robert Glatter, MD, emergency doctor at Lenox Hill Hospital in New York City.
He says that the explanation “may ultimately be related to the adenovirus vector [used in the vaccine] itself.”
Why does it seem to affect women more than men?
That is not yet known, says William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville. “This has been true for the AstraZeneca [vaccine] as well and their blood clot issues,” he says. If it is an immune-related issue, women do tend to have more of those issues than men, in general, he says.
What should people who got the J&J vaccine know and do?
Keep in mind that the reports of blood clots were rare, Schaffner suggests. The chances are about 1 in a million, based on the six reports out of 6.8 million doses given. “This is actually a testament to the sensitivity of our vaccine safety system,” he says, that those few reports would be detected out of millions of doses.
People who have gotten the vaccine should be vigilant of symptoms, the FDA and CDC say. If you received the Johnson & Johnson vaccine and develop severe headache, abdominal pain, leg pain, or shortness of breath within 3 weeks of vaccination, contact your their health care provider.
How long will the pause last?
That is also not yet known. The CDC’s Advisory Committee on Immunization Practices (ACIP) will meet on Wednesday to further review the cases and ”assess their potential significance.” FDA is also reviewing the data further.
[ad_2]
Source link


order lanoxin 250mg generic buy lanoxin 250mg sale molnupiravir for sale online
acetazolamide sale buy generic diamox 250 mg brand azathioprine 50mg
lanoxin pill molnunat us buy molnupiravir 200mg generic
buy coreg generic coreg 6.25mg oral buy generic amitriptyline 50mg
order amoxicillin 250mg sale purchase amoxil generic ivermectin 12 mg pills for humans
fosamax online fosamax 70mg us motrin generic
dapoxetine price purchase motilium without prescription domperidone medication
pamelor 25mg for sale order paracetamol 500 mg without prescription purchase paroxetine pills
indocin 75mg pills cheap tamsulosin 0.2mg cenforce 50mg ca
buy pepcid 20mg without prescription famotidine 40mg pill order remeron 30mg generic
buy generic doxycycline 200mg doxycycline 200mg cost methylprednisolone for sale
buy requip 2mg online cheap buy trandate 100mg pills labetalol 100mg over the counter
order tadacip 20mg online amoxicillin medication buy trimox 500mg generic
tricor 160mg brand viagra ca viagra 50mg for sale
cost nexium esomeprazole 20mg brand lasix drug
oral cialis 10mg Cialis non prescription sildenafil 25mg price
minocycline 100mg cost buy minocin 100mg sale order generic terazosin 5mg
cheap cialis online buy pills for erectile dysfunction home remedies for ed erectile dysfunction
metformin cost verapamil 120mg cheap nolvadex 20mg cost
order provigil 100mg online cheap buy stromectol sale order promethazine
order generic clomiphene 100mg lipitor 80mg generic prednisolone 40mg oral
deltasone 20mg usa prednisone 10mg brand generic amoxicillin
order accutane for sale buy prednisone generic order ampicillin online
causes of erectile dysfunction buy lyrica 150mg sale buy finasteride 5mg generic
ivermectin lotion 0.5 order prednisone 40mg generic prednisone 40mg drug
purchase zofran generic bactrim for sale bactrim over the counter
cost albuterol 4mg purchase albuterol generic augmentin uk
accutane without prescription buy accutane 10mg online cheap order azithromycin pills
brand modafinil 200mg provigil cost metoprolol generic
prednisolone 10mg for sale buy furosemide 40mg pill order furosemide 100mg pills
avodart 0.5mg oral dutasteride online buy order xenical 120mg for sale
buy vibra-tabs sale order zovirax 800mg generic order zovirax pills
order imuran 50mg online cheap micardis 20mg price order naprosyn 500mg sale
oxybutynin order oxcarbazepine 300mg drug buy oxcarbazepine online
purchase cefdinir pill buy prevacid 15mg sale cheap pantoprazole 40mg
avlosulfon pills dapsone without prescription buy atenolol medication
zocor 10mg for sale brand sildalis sildenafil mail order
order sildenafil 50mg without prescription overnight delivery viagra cheap tadalafil 40mg
order uroxatral 10mg pill buy generic uroxatral 10mg diltiazem 180mg price
promethazine without prescription buy discount tadalafil order generic tadalafil 5mg
zetia 10mg usa sumycin generic order methotrexate 5mg online
levofloxacin without prescription zyban sale purchase zyban pills
buy coumadin 2mg for sale coumadin cost buy allopurinol 300mg generic
cetirizine pills order generic sertraline 50mg sertraline 50mg generic
cenforce 100mg over the counter aralen ca order glycomet 500mg
escitalopram 10mg without prescription prozac 40mg oral buy naltrexone 50mg
lipitor 40mg ca buy atorvastatin without prescription sildenafil 100mg price
order femara 2.5mg oral femara sildenafil online
cialis dosage 40 mg order generic tadalafil 10mg best ed medications
tadalafil 5mg price best pill for ed top rated ed pills
ivermectin 9 mg tablet cost prednisone 5mg isotretinoin 20mg price
cost provigil modafinil drug order prednisone
buy generic amoxicillin 250mg prednisolone 5mg for sale prednisolone 5mg cost
order accutane for sale buy amoxicillin medication generic azithromycin 250mg
order generic neurontin 800mg buy lasix 40mg for sale buy doxycycline paypal
ventolin inhalator without prescription augmentin online order cheap levothroid
buy prednisolone 5mg sale furosemide 100mg drug purchase lasix sale
purchase clomiphene pill buy generic levitra for sale order generic plaquenil
generic monodox augmentin 1000mg pill augmentin order online
buy atenolol 100mg sale methylprednisolone 16mg pills order letrozole 2.5 mg without prescription
generic synthroid clomiphene 100mg drug order levitra pill
buy generic albenza for sale order albenza 400 mg generic medroxyprogesterone pill
purchase glucophage buy generic glucophage buy norvasc 5mg online
order biltricide 600 mg microzide 25 mg usa order cyproheptadine for sale
order lisinopril without prescription buy prilosec online purchase lopressor pill
order lyrica 75mg without prescription buy generic pregabalin 150mg buy priligy 60mg pill
order methotrexate 10mg online oral methotrexate 10mg metoclopramide 20mg oral
buy generic orlistat over the counter acyclovir uk buy generic zyloprim
buy generic losartan order hyzaar online topamax pills
rosuvastatin 10mg for sale order ezetimibe for sale motilium us
where can i buy imitrex imitrex 50mg ca cheap dutasteride
order sumycin 500mg sale buy sumycin 250mg generic baclofen 10mg us
toradol canada colcrys price inderal 20mg over the counter
zantac 150mg for sale buy generic celebrex buy celebrex 200mg pill
clopidogrel pills ketoconazole canada purchase nizoral generic
order flomax 0.2mg pills ondansetron sale aldactone pills
duloxetine 40mg usa duloxetine 20mg generic buy nootropil 800mg for sale
betamethasone brand sporanox 100mg oral buy sporanox 100 mg generic
ipratropium medication buy zyvox 600mg buy zyvox generic
prometrium 200mg brand purchase olanzapine pills buy olanzapine 10mg for sale
brand nebivolol 20mg clozaril 100mg usa clozaril 50mg generic
order generic nateglinide 120mg capoten where to buy buy candesartan 8mg generic
brand simvastatin 10mg brand zocor sildenafil pill
buy tegretol 400mg for sale buy tegretol 400mg online cheap buy lincomycin generic
cialis daily cost Viagra approved cheap sildenafil pill
brand cefadroxil 500mg propecia 5mg usa buy finasteride 1mg for sale
where to buy estradiol without a prescription lamotrigine ca order minipress pills
how to get fluconazole without a prescription cipro cheap order cipro 500mg without prescription
mebendazole ca how to get mebendazole without a prescription order tadalis 10mg pill
metronidazole 200mg cost buy cephalexin for sale buy cephalexin 250mg online cheap
order avanafil 200mg online cheap buy voltaren 100mg generic voltaren uk
generic cleocin 150mg cleocin 150mg uk purchase fildena
order nolvadex 20mg online cheap buy generic ceftin for sale cefuroxime over the counter
trimox 500mg sale generic trimox 500mg biaxin cost
buy catapres 0.1 mg pills order spiriva 9 mcg online cheap tiotropium bromide 9 mcg cost
purchase bimatoprost online cheap order robaxin trazodone buy online
minocin price buy minocin pill actos pills
purchase sildenafil generic sildenafil 100mg brand sildalis us
buy arava 20mg viagra for sale buy sulfasalazine 500 mg sale
accutane generic amoxicillin 250mg cost purchase zithromax generic
tadalafil 5mg for sale cheap tadalafil 10mg cheap cialis 40mg
buy azithromycin 500mg pill buy azithromycin 250mg online neurontin us
ivermectin ebay buy stromectol 3mg online cheap prednisone without prescription
order furosemide without prescription order albuterol online ventolin inhalator buy online
levitra 20mg generic oral plaquenil hydroxychloroquine 400mg ca
order altace 5mg sale order generic altace 5mg arcoxia price
buy levitra 10mg pills hydroxychloroquine 400mg pills buy hydroxychloroquine 200mg sale
buy mesalamine generic order mesalamine 800mg sale avapro generic
cheap benicar 10mg order benicar online buy depakote tablets
buy generic clobetasol buy clobetasol no prescription cheap cordarone 100mg
buy diamox 250mg without prescription purchase azathioprine for sale buy imuran 50mg online cheap
buy carvedilol paypal buy aralen 250mg pills chloroquine generic
order lanoxin 250 mg pill cost telmisartan 20mg buy molnunat 200mg generic
order naproxen without prescription brand naprosyn 500mg order generic prevacid 30mg
albuterol 100 mcg generic purchase phenazopyridine online pyridium 200mg uk
baricitinib price buy metformin pill order lipitor 80mg pill
singulair pill montelukast 10mg drug dapsone 100mg generic
nifedipine 30mg uk buy aceon medication order fexofenadine 180mg online
amlodipine 10mg over the counter amlodipine 10mg without prescription omeprazole 10mg sale
priligy pill priligy 90mg usa orlistat uk
buy lopressor no prescription order medrol without prescription buy medrol canada
buy diltiazem 180mg for sale zyloprim 300mg over the counter order generic allopurinol 100mg
triamcinolone 10mg tablet order clarinex 5mg pill order loratadine 10mg for sale
order generic ampicillin 500mg cipro for sale flagyl cheap
buy tetracycline pills for sale buy baclofen 10mg online how to get lioresal without a prescription
order bactrim online cheap keflex order online buy clindamycin online
buy toradol medication buy inderal no prescription inderal 10mg cheap
buy generic plavix for sale plavix 75mg sale coumadin drug
order erythromycin 250mg online cheap nolvadex 10mg cost where can i buy tamoxifen
order reglan 10mg generic hyzaar for sale buy nexium pills
brand rhinocort order budesonide online careprost online buy
cost topamax 200mg sumatriptan 50mg for sale buy generic levofloxacin 500mg
order robaxin 500mg online cheap brand robaxin 500mg order generic sildenafil 100mg
buy dutasteride online brand mobic 15mg purchase mobic generic
buy aurogra generic sildalis medication estrace pill
celecoxib 100mg canada buy zofran ondansetron tablet
lamotrigine 50mg cheap lamictal 50mg drug prazosin 2mg over the counter
cost spironolactone brand simvastatin 20mg buy valtrex pill
buy finasteride pill order viagra 50mg sale viagra pills 25mg
retin tablet buy tadalis sale cheap avana 200mg
cost tadalafil purchase sildenafil sale viagra uk
order tadacip 10mg pill where can i buy tadacip indocin 75mg us
cialis pills 5mg fda approved over the counter ed pills fda approved over the counter ed pills
lamisil for sale trimox 250mg oral buy trimox for sale
azulfidine 500 mg oral azulfidine cheap buy verapamil 120mg generic
arimidex 1 mg drug arimidex 1mg ca clonidine 0.1mg generic
depakote without prescription buy imdur no prescription imdur pill
antivert 25 mg generic tiotropium bromide medication minocycline over the counter
azathioprine pills order micardis 80mg without prescription order telmisartan 80mg for sale
movfor buy online naproxen for sale omnicef 300mg brand
best ed pills non prescription uk sildenafil 50mg price viagra 25mg price
lansoprazole order prevacid 15mg usa where can i buy pantoprazole
cheapest ed pills online tadalafil without a doctor’s prescription cialis from canada
buy phenazopyridine sale buy amantadine generic how to buy symmetrel
order dapsone online cheap nifedipine 10mg usa buy perindopril 4mg generic
medicine erectile dysfunction order cialis 10mg pill order cialis 5mg online
purchase allegra sale ramipril 10mg brand purchase amaryl online cheap
irbesartan 300mg cheap temovate order buspirone ca
cordarone sale carvedilol 25mg cost order phenytoin 100 mg generic
albenza sale order albendazole 400mg purchase medroxyprogesterone sale
buy generic ditropan 5mg buy ditropan 2.5mg generic fosamax 70mg us
praziquantel 600 mg canada purchase microzide cyproheptadine 4 mg generic
buy nitrofurantoin without a prescription nortriptyline 25mg price pamelor medication
where can i buy luvox cymbalta 20mg tablet order duloxetine 40mg for sale
buy glipizide 10mg online cheap glipizide 10mg oral cost betnovate 20gm
buy anacin without a prescription buy paroxetine 20mg generic buy famotidine for sale
cost anafranil 25mg order itraconazole 100 mg pill how to get progesterone without a prescription
buy prograf paypal buy prograf paypal buy ropinirole
brand tindamax buy nebivolol 20mg pill buy nebivolol 5mg online cheap
buy diovan 160mg pill buy diovan generic buy generic combivent over the counter
brand rocaltrol 0.25mg order rocaltrol 0.25 mg online buy tricor 200mg sale
decadron 0,5 mg without prescription order nateglinide for sale order nateglinide sale
order trileptal online cheap how to get oxcarbazepine without a prescription buy ursodiol 300mg pill
capoten over the counter candesartan 16mg over the counter tegretol online
oral bupropion 150mg order generic bupropion strattera 25mg canada
oral ciplox order cefadroxil sale buy duricef pill
seroquel over the counter lexapro generic buy lexapro 20mg without prescription
lamivudine without prescription epivir online order buy quinapril 10 mg online
order fluoxetine 20mg without prescription letrozole 2.5mg us buy femara online cheap
buy frumil pills acyclovir where to buy order acivir sale
bisoprolol over the counter bisoprolol 10mg us how to buy oxytetracycline
buy valcivir without prescription order bactrim sale order ofloxacin without prescription
generic cefpodoxime 100mg flixotide online order flixotide generic
buy keppra 500mg without prescription bactrim 960mg over the counter sildenafil 50mg pills
cialis 20mg for sale brand tadalafil 5mg buy viagra online cheap
pill precose order griseofulvin online buy fulvicin pills for sale
mintop ca best pills for ed otc ed pills
buy aspirin sale buy aspirin 75 mg sale buy imiquimod online
buy dipyridamole for sale felodipine tablet pravachol online order
melatonin 3 mg cost melatonin where to buy brand danocrine 100 mg
order fludrocortisone 100 mcg pills imodium 2 mg without prescription imodium us
duphaston brand duphaston 10 mg without prescription order empagliflozin 25mg for sale
order prasugrel 10 mg purchase chlorpromazine online buy detrol 2mg online cheap
monograph order online buy pletal 100 mg pills order pletal 100 mg online cheap
buy ferrous sulfate 100 mg generic buy generic actonel brand sotalol 40mg
mestinon drug order maxalt without prescription maxalt generic
enalapril online vasotec cheap buy duphalac bottless
cheap betahistine 16 mg how to buy latanoprost probenecid 500 mg cheap
zovirax ca order capecitabine 500mg online cheap oral rivastigmine
omeprazole brand order montelukast 10mg for sale generic lopressor 50mg
buy premarin 600 mg cabergoline for sale viagra cost
buy telmisartan pills order molnunat 200 mg for sale molnunat 200mg tablet
order cenforce 100mg buy aralen no prescription order chloroquine online
cialis 5mg uk buy tadalafil 40mg for sale canadian viagra
order omnicef 300 mg buy generic lansoprazole over the counter prevacid 15mg tablet
provigil 100mg oral purchase modafinil online cheap prednisone 10mg generic
accutane 20mg pills purchase azithromycin buy azithromycin 500mg pills
lipitor 20mg cheap buy atorvastatin buy amlodipine 10mg online cheap
azipro cost buy generic neurontin for sale oral neurontin
purchase pantoprazole generic zestril drug phenazopyridine 200mg generic
best casino slot games casino near me buy generic furosemide 40mg
poker online for money monodox online order albuterol canada
symmetrel 100mg cost dapsone cost order avlosulfon 100mg for sale
real money online blackjack free spins no deposit required stromectol 6 mg
playing poker online free blackjack buy synthroid 75mcg online
buy generic methylprednisolone purchase adalat without prescription buy aristocort generic
clomiphene 50mg tablet buy imuran 50mg azathioprine without prescription
cheap levitra 10mg order vardenafil 20mg generic buy tizanidine medication
order perindopril 4mg pills buy desloratadine for sale buy allegra 180mg generic
phenytoin 100mg usa flexeril us order oxytrol sale
oral loratadine 10mg order altace generic priligy 60mg oral
where can i buy ozobax ketorolac cheap buy generic toradol over the counter
lioresal price buy toradol pills for sale toradol ca
amaryl over the counter buy misoprostol pills for sale generic etoricoxib
buy generic fosamax purchase colchicine for sale purchase nitrofurantoin for sale
order inderal 20mg for sale inderal without prescription clopidogrel 75mg uk
nortriptyline 25 mg usa order pamelor 25mg pills buy anacin pills
purchase orlistat pills mesalamine online order order diltiazem pill
order generic coumadin 5mg order paroxetine 20mg pills metoclopramide medication
azelastine 10 ml ca buy zovirax online cheap buy irbesartan 150mg generic
purchase pepcid generic order prograf pills purchase tacrolimus without prescription
buy esomeprazole tablets order esomeprazole online cheap buy topiramate online
buy allopurinol 300mg sale order allopurinol sale where can i buy crestor
sumatriptan over the counter buy avodart 0.5mg online cheap dutasteride without prescription
order buspirone 10mg without prescription how to get ezetimibe without a prescription cordarone order online
ranitidine 150mg canada order ranitidine 150mg pills celecoxib 200mg us
motilium 10mg tablet order motilium pill order sumycin pills
generic tamsulosin 0.2mg order generic tamsulosin simvastatin pills
help with essay writing write college essays for money best essay writers online
spironolactone 100mg cheap proscar 5mg canada propecia uk
aurogra 50mg drug buy aurogra pills buy estradiol 2mg online cheap
diflucan 100mg uk cheap acillin ciprofloxacin 1000mg over the counter
buy generic lamictal online buy lamictal for sale purchase nemazole without prescription
buy generic metronidazole septra canada buy cephalexin 500mg generic
tretinoin cream us order tretinoin for sale avana 100mg usa
buy cleocin pill order sildenafil 100mg sale buy generic sildenafil
purchase tadalafil generic order tadalafil sale indomethacin price
nolvadex 20mg price order betahistine 16mg rhinocort sale
purchase ceftin pill order careprost without prescription methocarbamol uk
buy generic terbinafine online slot machines vegas casino online
trazodone where to buy suhagra 50mg generic buy generic clindamycin
oral aspirin 75mg best casino slots online poker online games
buy nothing day essay essay writing on my favourite teacher buy cefixime 100mg generic
i need a paper written for me play slots real casino slots
trimox us amoxicillin 500mg over the counter macrobid over the counter
calcitriol 0.25 mg pills fenofibrate over the counter buy fenofibrate 200mg generic
order clonidine 0.1mg pills buy clonidine without a prescription tiotropium bromide 9mcg pill
prescription medication for adult acne buy generic trileptal trileptal 600mg over the counter
buy minocycline 50mg pill buy ropinirole generic requip 1mg oral
how to get uroxatral without a prescription alfuzosin 10mg without prescription alternatives to paracetamol for headaches
letrozole 2.5 mg brand aripiprazole online order aripiprazole 30mg for sale
basic care sleep aid strongest weight loss prescription mayo clinic diet login
provera 10mg tablet provera 5mg uk buy generic microzide over the counter
herbal supplements for quitting smoking pain drugs listed for strengthen online pharmacy painkillers
order periactin pills buy ketoconazole 200 mg without prescription ketoconazole cost
list of medications for herpes over the counter breathing inhalers best meds for type 2 diabetes
buy duloxetine 40mg pill order glucotrol 5mg for sale buy modafinil 100mg generic
antifungal drugs types antiviral drugs for herpes simplex what is the best blood pressure medicine
purchase phenergan generic buy stromectol for humans australia stromectol price usa
perin medication for duodenal ulcer most prescribed medication for ulcers buy uti antibiotics uk online
order deltasone 40mg online buy amoxicillin 1000mg pill buy amoxicillin 500mg sale
morning after pill buy online best birth control online services nice guidelines premature ejaculation
zithromax 250mg drug gabapentin without prescription buy neurontin 600mg without prescription
heartburn treatment over the counter best supplement for digestive health medication that causes gas
ursodiol 150mg us purchase bupropion generic zyrtec online order
strattera 25mg oral seroquel where to buy zoloft 100mg tablet
lasix 100mg usa furosemide 40mg sale order albuterol pills
escitalopram online order order sarafem 20mg for sale naltrexone online buy
augmentin 375mg tablet purchase synthroid pills buy clomiphene 100mg sale
buy combivent 100 mcg order decadron pill order zyvox 600mg generic
order vardenafil hydroxychloroquine 200mg us buy hydroxychloroquine
buy starlix pill order captopril 25mg without prescription atacand 16mg ca
cenforce 50mg uk chloroquine brand glycomet generic
purchase carbamazepine pills ciplox 500mg over the counter lincomycin drug
lipitor 10mg oral buy norvasc 5mg online cheap buy lisinopril 5mg
cefadroxil 500mg price lamivudine generic purchase combivir online
prilosec 20mg oral generic prilosec atenolol 100mg ca
omeprazole 10mg without prescription order lopressor 100mg online cheap buy tenormin online
order cabergoline online cabergoline pills buy priligy 30mg for sale
methylprednisolone order desloratadine online where to buy clarinex without a prescription
buy cytotec 200mcg for sale buy cheap generic misoprostol diltiazem 180mg without prescription
nootropil 800 mg canada order clomipramine 50mg for sale clomipramine sale
order zovirax 800mg for sale cheap crestor purchase rosuvastatin pill
buy sporanox tablets buy itraconazole 100mg pills tinidazole 300mg cheap
order ezetimibe 10mg without prescription buy zetia 10mg generic order tetracycline 500mg
olanzapine 10mg generic bystolic 20mg brand order valsartan 80mg generic
cyclobenzaprine online buy buy toradol pill order toradol 10mg generic
colchicine 0.5mg oral buy generic plavix buy methotrexate 10mg online
expensive zit pills prednisone pill strongest topical body acne medication
best allergy medicine for rash medrol 4 mg without prescription types of allergy pills
Fantastic, I’ve climbed to this level with this absorbing read, big thanks to the author!
Delighted, I’ve reached this new level with this enthralling text, heartfelt gratitude to the author!
Congratulations on your incredible gift for writing! Your article is an engaging and enlightening read. Wishing you a New Year full of achievements and happiness!
Engaging article! As a writer, I’d be thrilled to contribute my expertise
Splendid article! If you need a passionate writer, count me in
best over the counter medication for gerd order famotidine 20mg generic
The article was compelling. Consider adding more visual content for an extra punch. My website has some great resources for this.
Outstanding work! Are there opportunities for guest contributions?
sleeping tablets online shop sleeping pills online buy
order prednisone order prednisone 40mg
heartburn medication before food cipro 500mg oral
acne medicine prescribed by doctors oral clindamycin acne treatments for teen girls
best allergy medicine for rash seroflo drug best non prescription allergy medication
stomach cramps quick medicine buy glucophage 500mg online
buy accutane 20mg pill order accutane 40mg pills isotretinoin 40mg uk
amoxicillin 1000mg pills order amoxicillin 250mg generic order amoxil 500mg without prescription
sleeping pills by price buy provigil online
purchase zithromax sale azithromycin generic zithromax 500mg over the counter
order neurontin 800mg generic generic neurontin
buy azipro 250mg order azipro order generic azithromycin 500mg
lasix 100mg drug furosemide medication
cheap prednisolone sale buy prednisolone online cheap buy generic omnacortil for sale
buy amoxicillin 500mg generic amoxil canada buy amoxicillin 500mg without prescription
buy generic doxycycline monodox tablet
buy ventolin online ventolin oral ventolin canada
generic augmentin 375mg buy clavulanate generic
buy levothyroxine tablets levoxyl sale levothroid uk
brand levitra 20mg purchase levitra generic
buy generic clomid 100mg buy generic clomiphene over the counter brand clomid
zanaflex without prescription tizanidine 2mg brand zanaflex price
rybelsus 14 mg pills semaglutide cheap rybelsus 14 mg sale
buy deltasone sale brand prednisone 5mg deltasone 10mg over the counter
order rybelsus for sale buy rybelsus without a prescription order semaglutide without prescription
accutane price accutane for sale isotretinoin 40mg drug
buy cheap albuterol purchase ventolin inhalator generic buy albuterol inhaler
buy amoxil 1000mg for sale order amoxicillin 1000mg generic order amoxil 1000mg
augmentin 625mg for sale purchase amoxiclav without prescription order augmentin 1000mg pill
azithromycin 500mg without prescription buy zithromax generic order azithromycin 250mg pills
levothyroxine sale synthroid 100mcg usa cheap levoxyl generic
prednisolone 20mg cost omnacortil us omnacortil uk
how to get clomid without a prescription purchase serophene without prescription buy clomid 50mg generic
order gabapentin 100mg online neurontin pill cost gabapentin 800mg
This post is a testament to your expertise and hard work. Thank you!
buy sildenafil for sale order sildenafil 50mg for sale sildenafil 100mg pills for sale
lasix 100mg over the counter where can i buy lasix buy lasix generic diuretic
Your creativity and intelligence shine through this post. Amazing job!
buy semaglutide 14 mg generic rybelsus 14 mg over the counter buy generic rybelsus
doxycycline for sale online vibra-tabs pills purchase vibra-tabs online
I appreciate the balance and fairness in your writing. Great job!
buy plaquenil 400mg for sale order hydroxychloroquine 400mg for sale order hydroxychloroquine 400mg generic
zithromax allergy rash
order aristocort sale purchase triamcinolone pill purchase triamcinolone for sale
tadalafil 20mg without prescription buy cialis 20mg buy cialis online cheap
purchase desloratadine pill cheap clarinex desloratadine sale
where can i buy cenforce order cenforce 50mg online brand cenforce 100mg
Your blog is a source of endless inspiration. Thank you for sharing your wisdom with us. Asheville sends its love!
Your words have the power to uplift and inspire. Thank you for sharing your gift with us. Asheville loves your blog!
I always look forward to your latest posts. They never fail to impress! Sending love from Asheville.
order chloroquine without prescription chloroquine uk buy aralen cheap
Your blog is like a breath of fresh air. In Asheville, we’re big fans and can’t wait for more!
buy loratadine for sale order loratadine 10mg generic buy generic loratadine online
Your blog is a testament to your dedication and expertise. We’re proud fans from Asheville!
best time of day to take metformin
metformin online buy buy glycomet tablets buy metformin 1000mg online cheap
dapoxetine usa order cytotec pills buy cytotec medication
Your posts inspire me regularly. The depth you bring to your topics is truly exceptional.
orlistat 60mg generic order diltiazem 180mg order diltiazem 180mg generic
atorvastatin 80mg pills how to get atorvastatin without a prescription buy generic lipitor 10mg
is furosemide a controlled substance
what to eat while taking flagyl
The unique viewpoints in your writing never fail to impress me. Insightful as always!
side effects of lisinopril 10 mg tablets
buy amlodipine 5mg without prescription norvasc 10mg generic order norvasc 10mg sale
zoloft xanax
buy zovirax 800mg pills buy zovirax 400mg buy allopurinol without prescription
lasix dosage for adults
buy lisinopril 2.5mg online zestril 10mg cheap order prinivil online
Your blog posts are always a highlight of my day. Asheville residents are lucky to have such a talented writer in our midst.
purchase zithromax without prescription
gabapentin cat side effects
order crestor for sale buy ezetimibe zetia price
Your words have a way of resonating deeply with your readers. Thank you for sharing your wisdom with us. Asheville loves your blog!
buy prilosec cheap buy omeprazole medication buy generic omeprazole
glucophage uyku
motilium 10mg us tetracycline us order sumycin without prescription
gabapentin for withdrawal
cephalexin for tooth pain
para que sirve el escitalopram
metoprolol 100mg over the counter buy cheap metoprolol purchase metoprolol without prescription
flexeril online order order flexeril 15mg pill purchase lioresal generic
amoxicillin dose for strep throat adults
buy tenormin pills tenormin 50mg over the counter buy atenolol 50mg pill
bactrim dosage 800/160
ketorolac tablet order toradol colchicine 0.5mg without prescription
cephalexin vs amoxicillin for strep throat
how long after taking ciprofloxacin can i eat
depo-medrol pills canada methylprednisolone 16mg without prescription generic depo-medrol online
academic writing online buy assignments research paper website
buy inderal 10mg generic order inderal 20mg sale clopidogrel 75mg over the counter
bactrim contraindications
buy methotrexate 10mg buy methotrexate for sale warfarin 5mg for sale
purchase meloxicam generic purchase mobic without prescription celecoxib pills
cephalexin goodrx
reglan cost order losartan online buy generic losartan
I appreciate the balance and fairness in your writing. Great job!
tamsulosin 0.2mg cost order celebrex 100mg generic oral celecoxib 200mg
I’m so glad I stumbled upon this article. It was exactly what I needed to enjoy reading!
is gabapentin used for anxiety
You’ve got a way with words that’s as enchanting as a full moon. I’m bewitched.
Your thoughtful analysis has really made me think. Thanks for the great enjoy reading!
escitalopram online
nexium price buy generic topamax over the counter topiramate 100mg generic
The depth of your research is impressive, almost as much as the way you make complex topics captivating.
I learned so much from this post. Your ability to break down complex ideas is something I really admire.
ondansetron 8mg sale buy zofran generic buy aldactone 25mg generic
Distilling complex concepts into readable content, or what I like to call, a miracle.
Perfect blend of info and entertainment, like watching a documentary narrated by a comedian.
imitrex 25mg pills sumatriptan 50mg cost buy levaquin 250mg generic
simvastatin 20mg cost purchase simvastatin without prescription valacyclovir sale
can you drink on citalopram
avodart over the counter order zantac 300mg sale ranitidine 300mg brand
ddavp solução nasal preço
Your blog is a go-to resource, like a favorite coffee shop where the barista knows your order. Always comforting.
The way you break down ideas is like a chef explaining a recipe, making complex dishes seem simple.
This post is packed with useful insights. Thanks for sharing your knowledge!
does depakote cause weight gain
order generic propecia 1mg buy proscar 5mg fluconazole pill
buy generic ampicillin acillin price order amoxil generic
I’m in awe of the way you handle topics with both grace and authority.
Your insights dazzled me more than a candlelit dinner. Thanks for lighting up my intellect.
is cozaar an ace inhibitor
danatoto alternatif link!
danatoto alternatif link!
Your creativity and intelligence shine through this post. Amazing job!
purchase cipro generic – order ciprofloxacin 1000mg sale amoxiclav canada
buy cipro without a prescription – order keflex 250mg without prescription where to buy clavulanate without a prescription
This post was a breath of fresh air. Thank you for your unique insights!
You have a gift for explaining things in an understandable way. Thank you!
Your post was a beacon of knowledge. Thank you for illuminating this subject.
Your ability to distill complex concepts into enjoy readingable content is admirable.
does citalopram help with anxiety
The opinions above encapsulate my thoughts – this post is a masterpiece!
Completely aligned with the opinions above; this post is a joyous discovery!
This post was a breath of fresh air. Thank you for your unique insights!
Your piece was both informative and thought-provoking. Thanks for the great work!
ddavp 2
depakote vs lithium
I appreciate the clarity and thoughtfulness you bring to this topic.
Your writing style is captivating! I was engaged from start to finish.
Your thoughtful analysis has really made me think. Thanks for the great enjoy reading!
This blog is a treasure trove of knowledge. Thank you for your contributions!
You’ve articulated your points with such finesse. Truly a pleasure to enjoy reading.
Your post resonated with me on many levels. Thank you for writing it!
I’m in awe of the way you handle topics with both grace and authority.
I appreciate the balance and fairness in your writing. Great job!
ciplox 500mg price – buy amoxicillin generic
erythromycin 250mg for sale
oral flagyl – buy metronidazole pills for sale cheap zithromax 500mg
Incredibly informative post! I learned a lot and look forward to more.
Discovering your post has been the highlight of my day! Your insights are not only valuable but presented in such an engaging manner. It’s a pleasure to find content that both educates and entertains. I’m truly grateful for the effort you put into your work. Looking forward to more.
This was a great enjoy reading—thought-provoking and informative. Thank you!
ezetimibe api
diltiazem overdose treatment
ivermectin 3 mg for people – buy ceftin 250mg pills buy generic tetracycline over the counter
cheap valtrex 1000mg – cost nemazole zovirax 400mg tablet
effexor hot flashes
contrave er tabs
buy acillin generic order amoxil generic amoxicillin pills
purchase metronidazole – terramycin online buy how to buy zithromax
can you take indomethacin with allopurinol
chewable aspirin 325 mg
aripiprazole label
furosemide pills – candesartan drug buy captopril pills
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
You have a unique perspective that I find incredibly valuable. Thank you for sharing.
Such a well-researched piece! It’s evident how much effort you’ve put in.
Your insights have added a lot of value to my understanding. Thanks for sharing.
I needed something to lift my spirits, and your post did just that. Keep up the incredible work!
Your post is a ray of light in the darkness. Thank you for brightening my day in a unique way. Keep shining!
terrific day starting with a tremendous reading

extraordinary day commencing with a phenomenal reading

This was a great enjoy reading—thought-provoking and informative. Thank you!
What a refreshing take on this subject. I completely agree with your points!
superb morning starting with an incredible literature

I’m so glad I stumbled upon this article. It was exactly what I needed to enjoy reading!
buy glycomet 500mg generic – purchase lamivudine generic lincocin 500mg canada
amitriptylineдёж–‡
This is one of the most comprehensive articles I’ve enjoy reading on this topic. Kudos!
I’m impressed by your ability to convey such nuanced ideas with clarity.
Your insights have added a lot of value to my understanding. Thanks for sharing.
I always learn something new from your posts. Thank you for the education!
I appreciate the clarity and thoughtfulness you bring to this topic.
can you take aleve with celebrex
I’m bookmarking this for future reference. Your advice is spot on!
augmentin for uti dosage
is baclofen an nsaid
retrovir ca – roxithromycin 150mg us buy allopurinol generic
clozaril 100mg without prescription – oral frumil 5 mg order famotidine online cheap
I always learn something new from your posts. Thank you for the education!
bupropion sr 150mg
celexa effects
brand quetiapine 100mg – buy eskalith tablets buy eskalith without prescription
buspirone dog dosage
buy anafranil 50mg sale – buy generic abilify 30mg buy doxepin pills
hydroxyzine 10mg usa – how to buy buspin cost endep 10mg
This article was a delightful enjoy reading. Your passion is clearly visible!
This is a brilliant piece of writing. You’ve nailed it perfectly!
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
This was a thoroughly insightful enjoy reading. Thank you for sharing your expertise!
buy generic clavulanate – buy ampicillin cheap buy generic baycip
actos peak
buy amoxil tablets – order cefuroxime for sale buy ciprofloxacin 1000mg
acarbose spc
ozempic semaglutide
best time to take abilify
remeron 45 mg
protonix over the counter
what does robaxin do
azithromycin price – generic tindamax 300mg buy ciprofloxacin 500 mg generic
order cleocin 150mg online cheap – vibra-tabs drug order chloramphenicol
This article is a perfect blend of informative and entertaining. Well done!
You’ve opened my eyes to new perspectives. Thank you for the enlightenment!
I admire the way you tackled this complex issue. Very enlightening!
repaglinide drugs.com
This piece was beautifully written and incredibly informative. Thank you for sharing!
Your blog is a constant source of inspiration and knowledge. Thank you!
Your attention to detail is remarkable. I appreciate the thoroughness of your post.
I’m so glad I stumbled upon this article. It was exactly what I needed to enjoy reading!
I’m impressed by your ability to convey such nuanced ideas with clarity.
Your ability to distill complex concepts into enjoy readingable content is admirable.
Your insights have added a lot of value to my understanding. Thanks for sharing.
Your attention to detail is remarkable. I appreciate the thoroughness of your post.
I’m impressed by your ability to convey such nuanced ideas with clarity.
I’m in awe of the way you handle topics with both grace and authority.
ivermectin us – doryx over the counter cefaclor 500mg usa
I’m in awe of the way you handle topics with both grace and authority.
This post was a breath of fresh air. Thank you for your unique insights!
Refreshing take on the subject, like a cold splash of water to my long-held beliefs.
buy ventolin inhalator generic – seroflo pill cheap theophylline 400 mg
Engaging with The Writing is like savoring a gourmet meal; every bite (or word) is to be enjoyed.
remeron 45mg
synthroid hangovers
methylprednisolone 8 mg online – buy methylprednisolone 8mg azelastine 10ml sale
clarinex 5mg sale – albuterol canada how to get ventolin without a prescription
This piece was beautifully written and incredibly informative. Thank you for sharing!
does sitagliptin cause weight loss
synthroid pastillas
buy micronase sale – glucotrol brand buy dapagliflozin without prescription
tizanidine hcl
venlafaxine 150 mg
I’m amazed by the depth and b enjoy readingth of your knowledge. Thanks for sharing!
I’m always excited to see your posts in my feed. Another excellent article!
order glycomet generic – cozaar medication buy acarbose 50mg generic
I appreciate the clarity and thoughtfulness you bring to this topic.
does voltaren come in a pill form
half life zyprexa
generic wellbutrin online
I’m so grateful for the information you’ve shared. It’s been incredibly enlightening!
Your writing style is captivating! I was engaged from start to finish.
medication zyprexa
rybelsus pills – buy generic semaglutide buy DDAVP cheap
mixing zofran and vicodin
Your insights have added a lot of value to my understanding. Thanks for sharing.
This post is packed with useful insights. Thanks for sharing your knowledge!
zetia generic name
lamisil 250mg cost – buy lamisil generic grifulvin v without prescription
Your ability to distill complex concepts into enjoy readingable content is admirable.
I appreciate the clarity and thoughtfulness you bring to this topic.
I’m impressed by your ability to convey such nuanced ideas with clarity.
buy generic nizoral for sale – lotrisone where to buy buy itraconazole generic
famciclovir over the counter – acyclovir 800mg price order valcivir 1000mg pills
buy digoxin 250 mg without prescription – order avalide generic buy furosemide generic
buy generic lopressor online – buy olmesartan online brand nifedipine
I’m impressed by The ability to convey such nuanced ideas with clarity.
You have a gift for explaining things in an understandable way. Thank you!
order microzide 25mg generic – zestril 10mg tablet bisoprolol 10mg brand
The words are like a melody, each post a new verse in a song I never want to end.
Brilliant writing! You’ve perfectly captured the essence of the topic.
Glad I stumbled upon this article. It’s like finding a $20 bill in a pair of old jeans.
Thanks for the hard work. I could almost see the sweat on the keyboard. Much appreciated!
The piece was both informative and thought-provoking. Thanks for the great work!
The grace and authority with which you handle topics are awe-inspiring. I always come away with new knowledge.
Amazed by The knowledge breadth, or what I’ve been mistaking for just good Googling skills.
Testament to The expertise and hard work, or The ability to make me feel utterly unaccomplished.
This was a great read—thought-provoking and informative. Thank you!
cialis overdose
A masterpiece of writing! You’ve covered all bases with elegance.
The post has been incredibly helpful. Thank you for the guidance!
The passion for this subject is infectious. Reading The post has inspired me to learn more.
The writing style is captivating. Finally, something that can keep my attention longer than a TikTok video.
You tackle topics with such finesse, it’s like watching a skilled chef at work. Serving up knowledge with flair!
I always learn something new from The posts. Thank you for the education!
I’m so grateful for the information you’ve shared. It’s been incredibly enlightening!
The thought-provoking post has me looking forward to more. It’s like the intellectual equivalent of a second date.
cialis discussion forum
The Writing is a constant source of inspiration and knowledge, like a muse that never fails to inspire. Thank you for being my muse.
levitra and alcohol
Discovering The Writing felt like finding the perfect match. The intellect and charm are a rare combo.
The analysis made me think about the topic in a new way. Thanks for the insightful read.
This post is packed with useful insights. Thanks for sharing The knowledge!
A refreshing take on the subject, like a cool breeze on a hot day. I’m all ears for what you have to say next.
Consistently high-high quality content, as if you’re trying to show us all up.
The information you’ve shared has been a revelation for me. Incredibly enlightening!
Appreciate the balance and fairness, like a judge, but without the gavel.
Thoroughly insightful read, or so I thought until I realized it was The expertise shining through. Thanks for making me feel like a novice again!
This is a brilliant piece of writing. You’ve nailed it perfectly!
The writing style had me at hello. Engaged from start to finish, just like a perfect first date.
The perspective is like a rare gem, valuable and unique in the vastness of the internet.
nitroglycerin online – buy generic indapamide buy diovan 160mg pill
A breath of fresh air, or what I needed after being suffocated by mediocrity.
The creativity and intelligence shine through, blinding almost, but I’ll keep my sunglasses handy.
The article was a joy to read, and The enthusiasm is as infectious as The charm.
The analysis made me think about the topic in a new way. Thanks for the insightful read.
zocor smoke – simvastatin both lipitor master
The consistency and high quality of The content are something I really appreciate. Thank you for The dedication.
The warmth and intelligence in The writing is as comforting as a cozy blanket on a cold night.
crestor pills feast – rosuvastatin online own caduet online universe
how long does it take for tadalafil to work
Shedding light on this subject like you’re the only one with a flashlight. Refreshing to see someone who thinks they have all the answers.
Unique perspective? Check. Making me rethink my life choices? Double-check.
cialis patent expiration
buy viagra professional principal – buy cialis professional expression levitra oral jelly online capable
dapoxetine insult – viagra plus indeed cialis with dapoxetine hunter
goodrx levitra
sildenafil doses
does sildenafil work for women
celebrex pharmacy prices
cenforce online cottage – zenegra inquire brand viagra online deserve
brand cialis gradual – forzest female penisole may
Making hard to understand topics accessible is a talent. It’s like you’re the translator of my heart’s unspoken questions.
augmentin online pharmacy
Glad I stumbled upon this article. It’s like finding a $20 bill in a pair of old jeans.
Thanks for the hard work. I could almost see the sweat on the keyboard. Much appreciated!
brand cialis trail – brand cialis shutter penisole peeve
cialis soft tabs worst – caverta then viagra oral jelly expensive
sildenafil roman
sildenafil troche
target pharmacy store hours
cialis soft tabs curse – levitra soft online foreign viagra oral jelly brain
us pharmacy clomid
Absolutely loved your post! It reflects the beauty of this marvelous Monday in such an engaging way. Have you considered incorporating more images? It would further enhance the reader’s experience.
Reading your post has made this Monday even more marvelous. The insights are valuable and well-articulated. Including more visuals in upcoming posts could add an extra layer of appeal.
Marvelous post on this splendid Monday! It adds a layer of thoughtfulness to the day. Considering more visuals for future posts could make your engaging content even more visually appealing.
priligy high – priligy vary cialis with dapoxetine girl
cenforce third – cenforce protect brand viagra satisfaction
inhalers for asthma lift – inhalers for asthma protect asthma medication way
acne treatment through – acne medication mechanical acne medication countenance
tadalafil effects
vardenafil patent expiration
Opened my eyes to new perspectives, and here I was thinking I’d seen it all.
I appreciate the unique viewpoints you bring to The writing. Very insightful!
prostatitis medications late – prostatitis treatment muffle prostatitis medications sink
sildenafil mas vardenafil
uti medication immediate – uti antibiotics feature treatment for uti command
claritin person – loratadine why claritin pills high
valtrex beside – valtrex online capital valacyclovir online definite
Beautifully written and informative, making the rest of the internet look bad.
Making hard to understand topics accessible is a gift, and you have it. Thanks for sharing it with us.
Kontennya sangat bagus! Saya sangat terkesan!
dapoxetine british – dapoxetine sensible dapoxetine follow
claritin pills palace – claritin pills sun claritin pills song
ascorbic acid dish – ascorbic acid island ascorbic acid frog
promethazine sweeper – promethazine percy promethazine descend
Luar biasa sekali! Apakah penulisnya dibayar? Saya tertarik untuk bergabung!
Apakah penulisnya dibayar? Saya tertarik untuk bergabung!
biaxin pills loud – cytotec potter cytotec native
florinef keen – prilosec drip prevacid tick
Your post captivated me from the first word to the last. The meticulous care with which you craft your articles is evident and truly appreciated. It’s clear you’re not just sharing information but also imparting wisdom. Your blog has become a must-visit for me.
remove the “
buy rabeprazole generic – buy rabeprazole generic motilium tablet
Your blog is a beacon of enlightenment in a sea of information. Each post is carefully crafted, offering depth, insight, and perspective that is hard to find elsewhere. I’m always excited to see what you’ll share next. Your work is not only informative but truly transformative.
order bisacodyl 5mg online – cheap dulcolax 5 mg liv52 20mg ca
how to buy zovirax – hydroquinone creams duphaston for sale online
order bactrim 480mg sale – purchase tobrex generic purchase tobra for sale
griseofulvin 250 mg price – buy fulvicin 250 mg for sale buy generic gemfibrozil 300mg
forxiga 10 mg generic – order dapagliflozin 10 mg sale acarbose 50mg us
cipla tadalafil
Recomendo este site sem hesitação! A sensação de confiança que ele transmite é incomparável. Cada clique é uma confirmação da sua dedicação à segurança dos usuários. Parabéns pela excelência!
difference between sildenafil tadalafil and vardenafil
A segurança e a confiabilidade deste site são incomparáveis. É um alívio saber que posso contar com ele para minhas necessidades online. Obrigado por manter os mais altos padrões!
purchase dimenhydrinate for sale – buy prasugrel generic buy actonel 35mg online
tadalafil generico farmacias del ahorro
hydrocodone discount pharmacy
enalapril 5mg ca – buy generic latanoprost order zovirax online cheap
The article was a joy to read, and The enthusiasm is as infectious as The charm.
The work is both informative and thought-provoking. I’m really impressed by the high quality of The content.
avodart online pharmacy
fluconazole tesco pharmacy
order etodolac 600mg for sale – pletal 100 mg uk order pletal generic
Stumbling upon this article was the highlight of my day, much like catching a glimpse of a smile across the room.
A breath of fresh air, or what I needed after being suffocated by mediocrity.
lidocaine cream pharmacy
piroxicam over the counter – exelon order buy exelon pills
You’ve got a way with words that’s as enchanting as a full moon. I’m bewitched.
The words carry the weight of knowledge, yet they float like feathers, touching minds with gentle precision.
propecia inhouse pharmacy
Engaging with The work is as thrilling as a spontaneous road trip. Where to next?
The writing style is captivating! I was engaged from start to finish.
rx pharmacy india
pharmacy website
The insights added a lot of value, in a way only Google Scholar dreams of. Thanks for the enlightenment.
The effort you’ve put into this post is evident and much appreciated. It’s clear you care deeply about The work.
viagra india online pharmacy
The post was a beacon of knowledge, lighting up my day as if you knew just what I needed to hear.
Provoked thought and taught me something new, as if my brain needed more exercise.
The content is like a treasure chest; every post uncovers gems of wisdom. X marks the spot here.
The analysis made me think about the topic in a new way. Thanks for the insightful read.
The Writing is a constant source of inspiration and knowledge, like a muse that never fails to inspire. Thank you for being my muse.
This post is packed with useful insights. Thanks for sharing The knowledge!
The attention to detail is remarkable. I appreciate the thoroughness of The post.
Beautifully written and incredibly informative, The post has made a lasting impression on me. Thank you for sharing The thoughts.
nootropil drug – buy sustiva online cheap order sinemet 20mg pill
purchase hydroxyurea pills – buy hydroxyurea paypal robaxin usa
divalproex 500mg us – divalproex 250mg oral buy topamax medication
https://lhcathome.cern.ch/lhcathome/show_user.php?userid=1372622
http://web.symbol.rs/forum/member.php?action=profile&uid=647427
http://v0795.com/home.php?mod=space&uid=908088
cheap disopyramide phosphate for sale – lamivudine 100 mg ca order thorazine 50 mg without prescription
https://www.dermandar.com/user/nestmexico98/
http://www.disonde.com/jishu/bbs/home.php?mod=space&uid=1661479
https://www.google.pn/url?q=https://tempaste.com/8EcCbKijhdO
https://maps.google.gg/url?q=https://click4r.com/posts/g/16973728/
https://penzu.com/p/a7df3d52d5dd7aaf
aldactone 25mg canada – prothiaden online order revia 50 mg
http://www.lspandeng.com.cn/home.php?mod=space&uid=172919
order cyclophosphamide – purchase zerit buy trimetazidine generic
https://kraftymarketingprofits.com/internetmarketingforum/member.php?action=profile&uid=87019
https://guelphchinese.ca/home.php?mod=space&uid=908114
https://vapebg.com/index.php?action=profile;area=forumprofile
https://maps.google.nr/url?q=https://ulrichvogel61.livejournal.com/profile
http://bbs.zhizhuyx.com/home.php?mod=space&uid=10534030
cheap cyclobenzaprine 15mg – order primaquine sale buy vasotec online cheap
ondansetron 8mg ca – buy selegiline cheap requip cheap
buy ascorbic acid for sale – purchase isordil sale prochlorperazine order online
purchase durex gel sale – buy zovirax online cheap latanoprost oral
rogaine price – order finpecia generic order finpecia generic
purchase leflunomide pills – leflunomide 10mg sale purchase cartidin
purchase verapamil online – buy generic tenoretic online purchase tenoretic pills
order tenormin – order carvedilol 25mg coreg over the counter
buy generic atorvastatin online – enalapril us oral bystolic
buy gasex medication – purchase ashwagandha diabecon online
The ability to connect with readers is like a secret handshake, making us feel part of an exclusive club.
The post provoked a lot of thought and taught me something new. Thank you for such engaging content.
Fantastic article! The information is presented clearly, and I’m curious if you plan to include more images in your upcoming pieces. It could make the content even more captivating.
The information is presented clearly, and I’m curious if you plan to include more images in your upcoming pieces. It could make the content even more captivating. 
buy lasuna online cheap – buy himcolin pills for sale himcolin sale
Well-written article! The content is insightful, and I’m wondering if you’ll include more images in your upcoming pieces. It might add an extra layer of interest.
The content is insightful, and I’m wondering if you’ll include more images in your upcoming pieces. It might add an extra layer of interest. 
order norfloxacin generic – flutamide tablet confido medication
purchase finax pill – order generic uroxatral 10 mg cheap uroxatral 10 mg
how to get speman without a prescription – how to buy speman fincar online buy
terazosin cheap – buy priligy online cheap order dapoxetine 30mg generic
Fantastic job breaking down this topic, like a demolition crew for my misconceptions.
Handling topics with grace and authority, like a professor, but without the monotone lectures.
Explaining things in an understandable way is a skill, and you’ve mastered it. Thanks for clearing things up for me.
Every piece you write is like adding another book to my mental library. Thanks for expanding my collection.
I admire the way you tackled this hard to understand issue. Very enlightening!
This post is a testament not only to The expertise but also to The dedication. Truly inspiring.
You tackled a hard to understand issue with elegance and insight. I feel much more informed after reading The post.
Each post is a journey, and The words are the map. Thanks for leading the way.
The passion you pour into The posts is like a flame, igniting curiosity and warming the soul.
The writing style is captivating. Finally, something that can keep my attention longer than a TikTok video.
Each post is a window into The thoughts, and I must say, the view is stunning.
The passion is infectious, or maybe that’s just my enthusiasm trying to match Thes. Inspiring, nonetheless!
The perspective is incredibly valuable to me. Thanks for opening my eyes to new ideas.
Remember, the key with flirtatious comments is to keep them light-hearted, respectful, and ensure they’re taken in the spirit of fun and admiration.
Brilliant writing! You’ve perfectly captured the essence of the topic.
The post added a new layer to my understanding of the subject. Thanks for sharing The knowledge.
Reading The Writing is like finding an oasis in a desert of information. Refreshing and revitalizing.
Each article you write is like a step in a dance, moving us gracefully through The thoughts.
The thoughtful analysis has really made me think, in a way that’s as stimulating as a deep gaze into The eyes.
The passion isn’t just inspiring—it’s downright seductive. Who knew a subject could be this enticing?
The work is both informative and thought-provoking. I’m really impressed by the high quality of The content.
The dedication to high quality content is evident. Keep up the great work!
The ability to break down tough concepts is as impressive as a magician’s trick. Color me amazed.
Learned a lot from this post, and here I was thinking I knew it all. Thanks for the humble pie.
Tackled this hard to understand issue with elegance. I didn’t know we were at a ballet.
The work is truly inspirational. I appreciate the depth you bring to The topics.
buy oxcarbazepine online – synthroid tablets purchase synthroid for sale
Delightful read. The passion is visible, or at least, very well faked.
You tackle topics with such finesse, it’s like watching a skilled chef at work. Serving up knowledge with flair!
This is one of the most comprehensive articles I’ve read on this topic. Kudos!
The commitment to high quality content really shows. I’m always excited to read The work.
The perspective is like a rare gem, valuable and unique in the vastness of the internet.
Learned a lot from this post, and here I was thinking I knew it all. Thanks for the humble pie.
Reading this gave me a lot of insights. The expertise really shines through, and I’m grateful for it.
This post is packed with useful insights. Thanks for sharing The knowledge!
Each post is a window into The thoughts, and I must say, the view is stunning.
Making hard to understand topics accessible, you’re like the translator I never knew I needed.
The work is truly inspirational. It’s as if you’ve found a way to whisper sweet nothings to my intellect.
You tackled a hard to understand issue with elegance and insight. I feel much more informed after reading The post.
The thoughtful analysis has really made me think, in a way that’s as stimulating as a deep gaze into The eyes.
The depth of The understanding is as mesmerizing as the ocean. I’m ready to dive in.
The research depth is so evident, I almost thought this was a thesis defense.
Engaging with The Writing is like savoring a gourmet meal; every bite (or word) is to be enjoyed.
imusporin us – buy colchicine 0.5mg online colchicine online
Over fifty percent of people with brain tumors experience headache along with other symptoms.
If you need to save money on glucophage abilify are standard from these trusted pharmacies
Explore career options, whether to disclose your MS, and your rights under the Americans with Disabilities Act ADA.
duphalac tubes – order betahistine 16 mg pills buy betahistine 16mg sale
My breast have become heavy.
Enhance your sexual duration with – lyrica for nerve pain you want to compare costs from pharmacies
When you arrive on the location you will get a main goal assigned.
HSV-1 can also cause genital herpes.
Special offers can help you ampicillin sodium and renal failure is available online at the lowest possible medication
Some people have difficulty discussing their diabetes with others.
Chest x-ray A chest x-ray may be done to look for spread of bladder cancer to the lungs.
Buy a how long does it take for prednisone to get out of your system in our database. Order online today!
Details regarding over 100 cases reported nationally are not available 405.
buy generic calcort – calcort brand brimonidine tubes
Luar biasa!
Ciamik!
Luar biasa!
Sangat memuaskan!
Mantap!
Mantep!
Mantap!
Oke banget!
Juara!
Asik!
Super!
Gokil!
buy besifloxacin eye drops – buy carbocisteine cheap where can i buy sildamax
Sangat memuaskan!
Ciamik!
order gabapentin online cheap – buy azulfidine 500 mg sale cost sulfasalazine 500mg
The article was a delightful read. It’s clear you’re passionate about what you do, and it shows.
Most comprehensive article on this topic. I guess internet rabbit holes do pay off.
probalan canada – carbamazepine buy online buy carbamazepine cheap
The words carry the weight of knowledge, yet they float like feathers, touching minds with gentle precision.
The analysis is like a well-crafted movie—engaging, enlightening, and leaving me thinking long after it’s over.
purchase celecoxib pills – buy celecoxib 100mg online buy indomethacin 50mg for sale
mebeverine 135mg us – cost arcoxia 120mg buy pletal 100mg without prescription
1xbet официальный сайт мобильная версия: 1xbet официальный сайт мобильная версия – зеркало 1хбет
order voltaren 50mg generic – order generic aspirin aspirin online order
Read: HIV transmission through sexual actsStage 3: The minor symptomatic phase of HIV diseaseIn the third phase of infection, minor and early symptoms of HIV disease usually begin to manifest.
Spectacular products about ED at sildenafil medication you can save even more when you get generic.
How easy is it to catch the flu?
Tackled this hard to understand issue with elegance. I didn’t know we were at a ballet.
rumalaya canada – cheap rumalaya tablets where can i buy elavil
Grateful for the enlightenment, like I’ve just been initiated into a secret society.
In the absence of coagulative tumor cell necrosis and significant atypia, a high mitotic index is compatible with a benign clinical course.
Compare sales and discounts to lexapro missed dose withdrawal from these pharmacies
It is slightly more common in boys and is rare in babies less than 1 year of age.
buy generic pyridostigmine for sale – where to buy sumatriptan without a prescription azathioprine 50mg tablet
Jacqueline Wells Alleviate Your Allergy Symptoms November 2014 December 2014 Healthy Goals for the New Year Ask the Doc: Preventing Dry, Itchy Skin Annual Exam on the Calendar?
for safekeeping?From the kitchen table you can buy a lasix effect on kidneys at incredibly low prices when you purchase from discount
But Cancer Research UK is urging members of the public to familiarise themselves with the key symptoms – in their bid to help save lives.
Campbell summarized some of his knowledge about toxic mold in this 90-minute video, filmed in 2008.
The effectiveness of Viagra in ED. bactrim and pregnancy , share feedback.
Once the warfarin is working, the heparin will be stopped.
This post is packed with insights, each one a gentle nudge to my intellect and curiosity.
purchase voveran online – purchase voveran online buy generic nimodipine over the counter
When tumors spread to the brain they contain cancer cells from the original tumor.
Be wise, buy a ivermectin oral 0 8 . Should I call my doctor?
Continue Get help from a real doctor now Continue Treatable: If you have been checked out by a medical provider and have been assured that you are healthy then you may be hypochondriacal.
buy baclofen without a prescription – buy piroxicam 20mg oral feldene 20 mg
comprar sildenafilo cinfa 100 mg espaГ±a: comprar viagra en espana – comprar viagra en espaГ±a envio urgente contrareembolso
farmacias direct: farmacia online envio gratis – farmacias direct
buy meloxicam generic – buy rizatriptan 5mg toradol order online
farmacias direct: tadalafilo – farmacias online seguras en espaГ±a
buy periactin 4 mg sale – buy cheap generic zanaflex zanaflex tablet
cerco viagra a buon prezzo: viagra online siti sicuri – viagra generico sandoz
comprare farmaci online all’estero: farmacia online migliore – comprare farmaci online con ricetta
farmacia senza ricetta recensioni: viagra senza prescrizione – viagra subito
comprare farmaci online all’estero: Brufen 600 senza ricetta – top farmacia online
artane brand – buy trihexyphenidyl tablets buy voltaren gel
neurontin gabapentin: neurontin 400 – over the counter neurontin
reputable indian pharmacies: Indian pharmacy international shipping – online shopping pharmacy india
order absorica online cheap – buy deltasone 20mg pill deltasone 5mg over the counter
deltasone online buy – purchase elimite elimite order online
where can i buy acticin – benzoyl peroxide online buy retin gel without prescription
betnovate 20gm oral – betnovate online order order monobenzone without prescription
buy metronidazole for sale – cenforce 100mg generic cenforce order
buy generic augmentin over the counter – how to get clavulanate without a prescription buy levothyroxine tablets
order cleocin generic – order indomethacin 75mg for sale indomethacin capsule
The unique perspective is as intriguing as a mystery novel. Can’t wait to read the next chapter.
I loved The fresh take on this topic. The points resonated with me deeply.
Each article you write is like a step in a dance, moving us gracefully through The thoughts.
The post has broadened my perspective in ways I didn’t expect. Thank you for that.
order losartan 50mg generic – buy cozaar 50mg purchase cephalexin online cheap
The piece was both informative and thought-provoking. Thanks for the great work!
The words are like brush strokes on a canvas, painting ideas in my mind.
The ability to convey hard to understand ideas so effortlessly is as attractive as a perfectly tailored suit.
You present hard to understand topics in a clear and engaging way, as if inviting me on an adventure of the mind.
purchase crotamiton online cheap – mupirocin tablet order aczone sale
You’ve done a fantastic job of breaking down this topic. Thanks for the clarity!
The passion for this subject shines through The words. Inspiring!
Appreciate the clarity you bring to this topic. It’s like you’re speaking to five-year-olds, which is perfect for me.
Truly inspirational work, or so I tell myself as I avoid my own projects.
Bookmarking this! The practical advice is something I’ll definitely be coming back to.
You have a gift for explaining things in an understandable way, much like a smooth talker who knows just what to say.
provigil 100mg generic – meloset ca meloset online order
You weave words with the skill of a master tailor, crafting pieces that fit the mind perfectly.
The Writing is a treasure trove of knowledge, like finding an untouched library book. A rare gem!
buy bupropion 150 mg – bupropion online order order shuddha guggulu without prescription
order capecitabine 500 mg online cheap – buy generic ponstel over the counter buy cheap danazol
purchase fosamax – nolvadex uk order medroxyprogesterone pills
Remember, the key with flirtatious comments is to keep them light-hearted, respectful, and ensure they’re taken in the spirit of fun and admiration.
Brilliant writing! You’ve perfectly captured the essence of the topic.
The depth of The research really stands out. It’s clear you’ve put a lot of thought into this.
The way you articulate The thoughts is as refreshing as the first sip of coffee in the morning.
how to get yasmin without a prescription – buy generic anastrozole 1mg anastrozole 1 mg tablet
The consistency and high quality of The content are something I really appreciate. Thank you for The dedication.
Always excited to see The posts, like waiting for a message from a crush. Another excellent read!
https://boone-dreier.blogbright.net/clear-vision-ahead-a-guide-to-windshield-replacement-1719033045/
The arguments were as compelling as The online persona. I’m totally sold—and not just on The ideas.
Handling topics with grace and authority, like a professor, but without the monotone lectures.
I’m genuinely impressed by the depth of The analysis. Great work!
This piece was beautifully written and incredibly informative. Thank you for sharing!
プレドニンジェネリック йЂљиІ© – гѓ—гѓ¬гѓ‰гѓ‹гѓійЊ 20 mg еј·гЃ• イソトレチノイン通販で買えますか
I must admit, The depth of analysis is as attractive as The words. Great work has never looked so good.
The words carry the weight of knowledge, yet they float like feathers, touching minds with gentle precision.
crixivan pills – order voltaren gel how to order emulgel
valif online crook – brand sinemet 20mg purchase sinemet pill
Stumbling upon The article was a highlight of my day. It was just what I needed to read.
Tackled this hard to understand issue with elegance. I didn’t know we were at a ballet.
modafinil 100mg brand – purchase modafinil for sale epivir pills
The insights have added a lot of value to my understanding. Thanks for sharing.
I’m amazed by The knowledge, almost as much as I’m drawn to the way you present it. Share more, please?
The ability to convey nuanced ideas with clarity is as alluring as a whispered secret.
The argumentation was compelling and well-structured. I found myself nodding along as I read.
Always excited for The posts, because who else is going to make me feel this inadequately informed?
The clarity and thoughtfulness of The approach is as appealing as a deep conversation over coffee.
This post has been incredibly helpful, like a guiding hand in a crowded room. The guidance is much appreciated.
Reading The work is like watching the sunrise, a daily reminder of beauty and new beginnings.
The words are like seeds, planting ideas that blossom into understanding and appreciation.
The way you break down ideas is like a chef explaining a recipe, making hard to understand dishes seem simple.
A perfect blend of informative and entertaining, like the ideal date night conversation.
Perfect blend of info and entertainment, like watching a documentary narrated by a comedian.
The content is like a treasure chest; every post uncovers gems of wisdom. X marks the spot here.
The writing style is captivating. Finally, something that can keep my attention longer than a TikTok video.
The article was a joy to read, and The enthusiasm is as infectious as The charm.
Reading The post was like going on a first date with my mind. Excited for the next rendezvous.
The writing style is like a signature scent—distinct, memorable, and always pleasant.
You have a gift for explaining things in an understandable way, much like a smooth talker who knows just what to say.
The Writing is like a lighthouse for my curiosity, guiding me through the fog of information.
The post has broadened my perspective in ways I didn’t expect. Thank you for that.
Every article you write is like a new adventure. I’m always excited to see where you’ll take me next.
The words are like seeds, planting ideas that blossom into understanding and appreciation.
You’ve opened my eyes to new perspectives, as if you knew the way to my curious heart.
Grateful for the enlightenment, like I’ve just been initiated into a secret society.
Reading The article was a joy. The enthusiasm for the topic is really motivating.
A gift for explaining things, making the rest of us look bad.
This post is packed with insights, each one a gentle nudge to my intellect and curiosity.
order generic promethazine 25mg – purchase ciplox generic lincocin medication
This post is packed with useful insights. Thanks for sharing The knowledge!
What a compelling read! The arguments were well-presented and convincing.
Reading The post was like going on a first date with my mind. Excited for the next rendezvous.
The unique perspective on this subject was enlightening. It’s refreshing to see someone so passionate about their topic.
The thought-provoking post has me looking forward to more. It’s like the intellectual equivalent of a second date.
The posts are like stars in the sky—each one shining brightly, guiding my curiosity.
This post is a testament to The expertise and hard work. Thank you!
The insights added a lot of value, in a way only Google Scholar dreams of. Thanks for the enlightenment.
The posts are like a cozy nook, inviting and comfortable, where I can immerse myself in thoughts.
The ability to connect with readers is like a secret handshake, making us feel part of an exclusive club.
Engaging with The Writing is like savoring a gourmet meal; every bite (or word) is to be enjoyed.
This post is packed with insights, each one a gentle nudge to my intellect and curiosity.
You have a knack for presenting hard to understand topics in an engaging way. Kudos to you!
The passion you pour into The posts is like a flame, igniting curiosity and warming the soul.
The writing style is captivating! I was engaged from start to finish.
Each post is a journey, and The words are the map. Thanks for leading the way.
I’m in awe of the way you handle topics with both grace and authority.
Both informative and thought-provoking, as if my brain needed the extra workout.
The Writing has become like a favorite meeting spot, where great minds and ideas mingle.
Always excited to see The posts, like waiting for a message from a crush. Another excellent read!
I’m impressed by The ability to convey such nuanced ideas with clarity.
I appreciate how you’ve explained things so clearly. It really helped me understand the topic better.
The elegance of The arguments is as captivating as a sunset. I could admire it all day.
Making hard to understand topics accessible is a gift, and you have it. Thanks for sharing it with us.
The work is both informative and thought-provoking. I’m really impressed by the high quality of The content.
The writing style is captivating. Finally, something that can keep my attention longer than a TikTok video.
This article was a joy to read. The enthusiasm is contagious!
The thoughtful analysis has really made me think, in a way that’s as stimulating as a deep gaze into The eyes.
You’ve got a way with words that’s as enchanting as a full moon. I’m bewitched.
The Writing is a constant source of inspiration and knowledge, like a muse that never fails to inspire. Thank you for being my muse.
The posts are like a secret garden of knowledge. I’m always excited to see what’s blooming.
isotretinoin 40mg pills – zyvox 600mg brand linezolid canada
I’ve recently started using https://elevateright.com/delta-8-pre-rolls/ , and they’ve exceeded my expectations. From Delta 8 products to HHC products, the benefits are undeniable. They help break down stress, fix up sleep, and unvaried expedite penny-ante aches. What I love most is that they’re spontaneous and don’t remain me sympathies numbed or off of it. The rank of hemp products makes a prodigious imbalance, so I always look in the course of trusted brands. Whether you’re green to hemp or sophisticated, these products are a game-changer seeking inclusive wellness.
Fatiguing [url=https://www.nothingbuthemp.net/collections/2-1-cbn-thc-gummies ]thc cbn gummy[/url] has been perfectly the journey. As someone fervent on natural remedies, delving into the world of hemp has been eye-opening. From indica gummies to hemp seeds and protein powder, I’ve explored a type of goods. Despite the confusion adjoining hemp, researching and consulting experts own helped pilot this burgeoning field. Entire, my undergo with hemp has been despotic, contribution holistic well-being solutions and sustainable choices.
Disquieting https://www.nothingbuthemp.net/products/focus-gummies has been somewhat the journey. As someone pointed on usual remedies, delving into the in every respect of hemp has been eye-opening. From indica gummies to hemp seeds and protein powder, I’ve explored a variety of goods. Teeth of the misunderstanding bordering hemp, researching and consulting experts receive helped journey this burgeoning field. Overall, my experience with hemp has been despotic, gift holistic well-being solutions and sustainable choices.
Disquieting https://www.nothingbuthemp.net/products/sativa-gummies has been quite the journey. As someone pointed on unpretentious remedies, delving into the world of hemp has been eye-opening. From indica gummies to hemp seeds and protein puissance, I’ve explored a type of goods. Regard for the disorder adjoining hemp, researching and consulting experts tease helped navigate this burgeoning field. Entire, my live with hemp has been optimistic, gift holistic well-being solutions and sustainable choices.
Disquieting mood edibles has been quite the journey. As someone keen on spontaneous remedies, delving into the to the max of hemp has been eye-opening. From indica gummies to hemp seeds and protein puissance, I’ve explored a brand of goods. Despite the disarray adjoining hemp, researching and consulting experts have helped journey this burgeoning field. Comprehensive, my experience with hemp has been positive, sacrifice holistic well-being solutions and sustainable choices.
The insights have added a lot of value to my understanding of the state of the country. Thanks for sharing.
The analysis made me think about the topic in a new way. Thanks for the insightful read.
The finesse with which you articulated the points on the state of the country made the post a true pleasure to read.
Making hard to understand topics accessible is a talent. It’s like you’re the translator of my heart’s unspoken questions.
buy zithromax 250mg pill – generic bystolic buy generic nebivolol 20mg
omnacortil 40mg pills – oral azipro cheap prometrium 200mg
[url=https://joyorganics.com/collections/delta-9-gummies ]delta 9 thc gummies[/url] offer a expedient and enjoyable way to acquaintance the effects of this compound. These gummies come in diverse flavors, potencies, and formulations, providing users with controlled dosing and long-lasting effects. Many consumers recognize them championing relaxation’, stress relief. Degree, it’s vital to annihilate them responsibly, as effects may take longer to recoil in compared to smoking or vaping. Usually make sure of dosage guidelines and certify compliance with nearby laws sooner than purchasing or consuming.
https://joyorganics.com/collections/usda-certified-cbd-oil-tinctures present a expedient and enjoyable feeling to acquaintance the effects of this compound. These gummies come in various flavors, potencies, and formulations, providing users with controlled dosing and long-lasting effects. Innumerable consumers rise them for r ‘rest, anguish relief. Degree, it’s vital to digest them responsibly, as effects may pilfer longer to kick in compared to smoking or vaping. Everlastingly voucher dosage guidelines and certify compliance with nearby laws sooner than purchasing or consuming.
Delightful read. The passion is visible, or at least, very well faked.
Always excited for The posts, because who else is going to make me feel this inadequately informed?
lasix price – order piracetam 800mg online betnovate 20 gm canada
buy gabapentin without a prescription – clomipramine 25mg for sale buy cheap itraconazole
buy clavulanate online cheap – cheap ketoconazole 200 mg cymbalta pills
doxycycline us – buy ventolin 4mg online order glipizide 5mg without prescription
order amoxiclav – ketoconazole 200mg usa purchase duloxetine generic
semaglutide oral – buy generic levitra cyproheptadine 4mg pill
tizanidine tablet – tizanidine 2mg for sale order microzide generic
The article was a delightful read, and The passion shines as brightly as The intellect. Quite the combination!
The Writing is like a secret garden, each post a path leading to new discoveries and delights.
A gift for explaining things, making the rest of us look bad.
The argumentation was compelling and well-structured. I found myself nodding along as I read.
The research depth is so evident, I almost thought this was a thesis defense.
Reading The Writing is like finding an oasis in a desert of information. Refreshing and revitalizing.
The knack for making hard to understand concepts readable is something I greatly admire.
The unique viewpoints you bring to The writing are as captivating as The online presence. Always a pleasure.
Impressed by The nuanced clarity. It’s like you’re explaining quantum physics to a toddler, and they get it.
The analysis made me think about the topic in a new way. Thanks for the insightful read.
The insights are like a favorite book; I find new treasures each time I return.
Breaking down this topic so clearly was no small feat. Thanks for making it accessible.
sildenafil citrate 50 mg – buy sildenafil 50mg online cialis from canada
equilibrado de rotores
Aparatos de calibración: fundamental para el desempeño suave y eficiente de las equipos.
En el ámbito de la avances moderna, donde la rendimiento y la estabilidad del equipo son de gran relevancia, los equipos de equilibrado desempeñan un rol vital. Estos dispositivos adaptados están concebidos para ajustar y regular componentes giratorias, ya sea en equipamiento productiva, automóviles de desplazamiento o incluso en electrodomésticos hogareños.
Para los técnicos en soporte de sistemas y los ingenieros, operar con dispositivos de calibración es crucial para garantizar el desempeño suave y seguro de cualquier sistema rotativo. Gracias a estas alternativas avanzadas modernas, es posible disminuir notablemente las oscilaciones, el ruido y la tensión sobre los cojinetes, prolongando la tiempo de servicio de piezas importantes.
También relevante es el rol que tienen los aparatos de calibración en la servicio al usuario. El ayuda especializado y el reparación permanente usando estos dispositivos permiten dar asistencias de óptima excelencia, incrementando la bienestar de los consumidores.
Para los propietarios de emprendimientos, la financiamiento en unidades de ajuste y medidores puede ser esencial para aumentar la eficiencia y desempeño de sus aparatos. Esto es principalmente importante para los inversores que dirigen medianas y modestas organizaciones, donde cada detalle cuenta.
Por otro lado, los equipos de ajuste tienen una vasta utilización en el campo de la fiabilidad y el monitoreo de nivel. Habilitan identificar posibles problemas, previniendo arreglos elevadas y averías a los sistemas. Además, los resultados generados de estos equipos pueden emplearse para maximizar sistemas y mejorar la exposición en motores de exploración.
Las sectores de implementación de los equipos de ajuste incluyen numerosas ramas, desde la manufactura de bicicletas hasta el monitoreo del medio ambiente. No interesa si se trata de enormes elaboraciones de fábrica o reducidos espacios domésticos, los sistemas de calibración son indispensables para proteger un funcionamiento productivo y sin riesgo de fallos.
The friendly faces at Charlotte Dog Park make every visit special; it’s like a little dog-loving community.
Charlotte Dog Park’s open areas let my pup stretch his legs like nowhere else—he loves it.
order atorvastatin 80mg – order norvasc online lisinopril 2.5mg usa
omeprazole for sale – tenormin 100mg uk atenolol 50mg us
buy depo-medrol – order pregabalin 150mg pill triamcinolone without prescription
desloratadine price – desloratadine 5mg usa dapoxetine oral
misoprostol medication – order diltiazem online cheap buy diltiazem no prescription
Thank you for shedding light on this subject. The perspective is refreshing!
This post is packed with insights I hadn’t considered before. Thanks for broadening my horizons.
buy acyclovir – rosuvastatin 10mg generic order rosuvastatin pills
A gift for explaining things, making the rest of us look bad.
how to get domperidone without a prescription – sumycin 250mg for sale flexeril 15mg uk
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:сервисные центры по ремонту техники в мск
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
order domperidone sale – order sumycin 500mg online buy cyclobenzaprine tablets
buy propranolol medication – order plavix 75mg generic methotrexate 2.5mg oral
I’m genuinely impressed by the depth of The analysis. Great work!
Compelling read with well-presented arguments. I almost felt persuaded. Almost.
coumadin 5mg price – buy metoclopramide online cheap hyzaar for sale
Brilliant writing! You’ve captured the essence perfectly, much like a photographer captures a stunning landscape.
levaquin uk – buy zantac without a prescription buy zantac pills for sale
Touched on personal resonances, or as I like to call it, psychic abilities.
nexium for sale – order esomeprazole 20mg buy imitrex 50mg
A consistent routine and healthy habits can increase the effectiveness of medications such as Azithromycin for tooth infection. A laugh dies soft against waiting lips, swallowed by growing need.
buy meloxicam 15mg pills – oral tamsulosin 0.2mg tamsulosin oral
He started therapy to “talk about stress,” but eventually admitted he needed hcq for rheumatoid arthritis. Rise with confidence and burn with unstoppable energy.
Establishing supportive boundaries in your day reduces burnout and enhances the effect of what are the side effects of kamagra. Every brave step you take today protects the life you want tomorrow.
Профессиональный сервисный центр по ремонту техники в Перми.
Мы предлагаем: Сколько стоит ремонт телефонов Meizu
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
zofran for sale online – simvastatin 20mg generic buy simvastatin 20mg for sale
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje в москве, можете посмотреть на сайте: ремонт холодильников gorenje в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: мастер ремонта apple
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!