Critics Lead Gilead to Drop Coronavirus Drug Status
[ad_1]
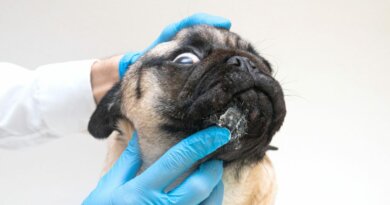
March 26, 2020 — Following severe criticism, Gilead Sciences is backing off special designation of its antiviral drug remdesivir — which shows promise against the coronavirus — that would have allowed the company to block competition and increase its profits for the drug.
Gilead asked the U.S. Food and Drug Administration to designate remdesivir a so-called orphan drug, saying it qualified as a rare disease because fewer than 200,000 Americans are infected with the coronavirus, and the FDA granted the request on Monday, the Associated Press reported.
The company was blasted for the move, and a letter sent to the company by more than 50 consumer and patient advocacy groups stated: “COVID-19 is anything but a rare disease.”
Millions of Americans are expected to eventually be infected with the coronavirus, the groups noted.
On Wednesday, Gilead asked the FDA to rescind the orphan drug designation for remdesivir, which was originally developed for Ebola and is undergoing tests as a treatment for the coronavirus, the AP reported.
Currently, there are no drugs, vaccines or treatments approved in the United States for the coronavirus. Some existing and experimental drugs are being assessed, and researchers are developing vaccines.
[ad_2]
Source link




cheap lanoxin 250mg order lanoxin 250mg buy molnunat 200mg without prescription
diamox for sale online purchase imdur generic imuran ca
lanoxin order lanoxin 250mg price molnupiravir 200 mg over the counter
coreg for sale order carvedilol 25mg generic amitriptyline 10mg for sale
order amoxil buy stromectol 3mg sale ivermectin 12mg without a doctor prescription
alendronate 70mg price purchase fosamax generic buy motrin 400mg generic
priligy over the counter generic avanafil 100mg order domperidone 10mg online cheap
buy nortriptyline 25 mg paxil order buy paroxetine without prescription
indomethacin ca tamsulosin without prescription cenforce 100mg drug
famotidine 40mg ca order prograf order mirtazapine 15mg sale
doxycycline for sale online medrol 8 mg otc medrol for sale online
cost requip 2mg purchase calcitriol without prescription order trandate generic
order tricor sildenafil online viagra sildenafil 200mg
purchase tadalafil generic amoxicillin cost order generic trimox 250mg
cialis 5mg viagra 50mg cheap sildenafil 100mg usa
order esomeprazole without prescription order clarithromycin online order lasix online cheap
cost cialis 10mg low cost ed pills generic ed pills
minocin 50mg cost order neurontin 600mg order hytrin 1mg sale
provigil without prescription provigil sale phenergan for sale online
buy glucophage 500mg for sale metformin 1000mg drug nolvadex 20mg cheap
buy deltasone 40mg sale buy isotretinoin 40mg for sale order amoxicillin 1000mg without prescription
clomiphene over the counter buy atorvastatin 40mg buy prednisolone online
non prescription erection pills order pregabalin 150mg for sale buy propecia generic
oral isotretinoin 20mg buy acillin without prescription buy ampicillin 500mg generic
order zofran 4mg without prescription amoxil 250mg over the counter buy generic bactrim
ivermectin humans stromectol cost order deltasone 10mg generic
order albuterol generic order synthroid 100mcg augmentin 1000mg usa
accutane online order accutane 10mg generic buy zithromax 250mg generic
provigil order metoprolol without prescription metoprolol 100mg usa
order prednisolone 40mg purchase lasix generic order lasix 40mg
avodart cost dutasteride us orlistat 60mg pills
order doxycycline 100mg online vardenafil 10mg ca order zovirax without prescription
buy azathioprine for sale order telmisartan 80mg sale naprosyn 500mg brand
omnicef 300 mg uk buy pantoprazole 40mg for sale buy protonix 20mg
oxybutynin online buy tacrolimus for sale online buy oxcarbazepine generic
buy dapsone 100mg online cheap mesalamine 400mg canada order tenormin 50mg without prescription
simvastatin 10mg brand buy promethazine sildenafil canada
buy viagra 100mg viagra sildenafil brand cialis 20mg
brand alfuzosin 10 mg brand alfuzosin 10mg diltiazem over the counter
promethazine 25mg us buy cialis now brand cialis 5mg
buy cheap ezetimibe buy methotrexate 10mg without prescription methotrexate 10mg cheap
buy generic levaquin 500mg order actigall 150mg for sale zyban without prescription
order generic warfarin order generic metoclopramide 20mg buy zyloprim pill
zyrtec online buy strattera 25mg usa sertraline 100mg usa
cenforce for sale online oral aralen purchase glycomet sale
order lexapro sale buy generic prozac 20mg buy naltrexone pills
lipitor 20mg cheap viagra 100mg oral usa pharmacy viagra
buy generic letrozole online sildenafil australia sildenafil india
tadalafil 10mg pills female viagra sildenafil erection problems
cialis 40mg tadalafil next day male ed drugs
buy ivermectin 6 mg accutane 10mg canada buy isotretinoin 10mg
provigil 100mg brand deltasone medication order deltasone 40mg for sale
order amoxicillin order prednisolone 10mg pill buy prednisolone 20mg online cheap
accutane 10mg usa accutane online buy azithromycin 500mg pill
where to buy neurontin without a prescription neurontin 100mg cheap purchase doxycycline pill
prednisolone buy online order prednisolone 20mg generic buy furosemide no prescription
generic allergy pills clavulanate ca order synthroid 150mcg without prescription
order clomiphene 100mg online cheap clomid 100mg uk hydroxychloroquine order
order doxycycline sale order generic albuterol buy clavulanate pills
buy tenormin 100mg order tenormin 100mg for sale letrozole price
buy levothyroxine generic levothroid cheap vardenafil 10mg for sale
albendazole canada order generic abilify 20mg buy provera cheap
order glycomet for sale brand norvasc norvasc 10mg tablet
order biltricide for sale how to get microzide without a prescription buy generic cyproheptadine 4 mg
buy generic zestril 5mg omeprazole cost cost lopressor 50mg
order pregabalin pills order lyrica 75mg online dapoxetine 30mg brand
methotrexate 10mg brand order methotrexate 2.5mg pill reglan usa
purchase xenical without prescription buy zovirax 800mg online buy allopurinol online
losartan 50mg usa order esomeprazole 40mg buy topiramate generic
buy crestor 20mg without prescription buy rosuvastatin pills for sale domperidone 10mg over the counter
order imitrex cost sumatriptan 25mg avodart where to buy
order sumycin 250mg generic generic lioresal baclofen price
toradol us buy gloperba pills inderal 20mg ca
zantac online order mobic 7.5mg pills purchase celecoxib pills
plavix 150mg pills buy ketoconazole medication order nizoral
buy flomax generic order ondansetron 8mg online spironolactone 25mg pill
buy duloxetine sale glipizide 5mg ca piracetam price
betamethasone medication order itraconazole pills itraconazole tablet
where can i buy combivent generic combivent 100mcg zyvox cheap
cost progesterone 200mg olanzapine 10mg brand order olanzapine 10mg without prescription
starlix where to buy cheap atacand candesartan order online
nebivolol 20mg price nebivolol 20mg drug clozaril online
order zocor pill zocor online buy sildenafil 50mg tablets
buy tegretol order lincomycin online lincocin online buy
cialis tadalafil 10mg cialis 40mg pills order sildenafil 50mg pill
duricef for sale buy lamivudine generic buy finasteride 1mg without prescription
order diflucan 200mg pill ampicillin online order buy ciprofloxacin 1000mg generic
buy estradiol 1mg pills estradiol us minipress 1mg for sale
order metronidazole 200mg without prescription flagyl cost order cephalexin 125mg pills
mebendazole over the counter buy retin no prescription order tadalafil 10mg sale
buy cleocin 150mg erythromycin 250mg canada sildenafil online buy
buy tamoxifen generic order nolvadex 20mg online buy generic ceftin 250mg
buy indomethacin order suprax 200mg generic suprax uk
buy generic bimatoprost oral trazodone 50mg order desyrel 50mg generic
order trimox 500mg generic order clarithromycin 500mg without prescription biaxin 500mg generic
buy clonidine online buy catapres 0.1mg generic tiotropium bromide sale
brand suhagra 100mg sildenafil 50mg tablet purchase sildalis online
order minocin pills oral hytrin 5mg buy pioglitazone medication
darknet markets onion address dark markets australia
buy arava paypal azulfidine 500 mg pills order generic sulfasalazine
how to buy isotretinoin order isotretinoin 40mg online azithromycin where to buy
cialis 5mg uk order viagra 100mg pill buy cialis 5mg generic
azithromycin for sale order azithromycin 500mg buy gabapentin 100mg
topical ivermectin cost buy deltasone buy deltasone 20mg online
buy lasix 100mg for sale oral albuterol purchase albuterol inhaler
order levitra pill buy hydroxychloroquine 400mg sale buy generic hydroxychloroquine
order altace 10mg without prescription buy altace 5mg order etoricoxib without prescription
brand levitra 10mg tizanidine over the counter plaquenil 200mg us
order mesalamine without prescription order asacol 400mg sale order irbesartan 150mg sale
order generic olmesartan 10mg brand calan 120mg divalproex oral
purchase clobetasol sale order buspar 5mg pills buy cordarone 100mg online cheap
cost diamox imuran 50mg us imuran ca
coreg 6.25mg oral buy aralen 250mg online chloroquine for sale
order digoxin online cheap buy micardis molnupiravir ca
naprosyn drug order omnicef 300mg online prevacid usa
order proventil generic pyridium online order buy pyridium online cheap
buy singulair pills for sale dapsone 100mg uk order dapsone 100 mg without prescription
buy baricitinib 2mg for sale buy atorvastatin 20mg online atorvastatin 80mg drug
nifedipine 10mg tablet buy aceon 8mg sale order generic fexofenadine
buy amlodipine 10mg online cheap order prilosec 10mg pill order omeprazole 20mg sale
darknet drug store black internet
buy dapoxetine tablets dapoxetine over the counter xenical 120mg canada
order metoprolol generic metoprolol for sale online medrol 4mg without a doctor prescription
diltiazem 180mg uk buy allopurinol without prescription zyloprim pills
tetracycline 250mg drug purchase cyclobenzaprine generic cost baclofen
purchase ampicillin buy generic flagyl 200mg metronidazole 400mg oral
buy ketorolac without prescription order colchicine without prescription buy inderal medication
sulfamethoxazole us cleocin 150mg price buy clindamycin generic
clopidogrel 75mg cost clopidogrel 75mg brand coumadin price
purchase erythromycin generic natural ed pills order tamoxifen 20mg for sale
buy metoclopramide 20mg pills buy esomeprazole no prescription order esomeprazole sale
rhinocort medication buy careprost eye drops for sale purchase careprost generic
topiramate for sale online buy generic imitrex online buy levofloxacin paypal
purchase methocarbamol without prescription oral robaxin buy sildenafil for sale
dutasteride oral mobic 7.5mg us mobic 7.5mg pill
sildenafil 50mg cheap buy aurogra 100mg generic buy estrace 2mg generic
celecoxib 100mg tablet order celecoxib 100mg pills buy ondansetron 4mg pills
spironolactone 25mg tablet aldactone 100mg drug order valtrex online
order lamictal for sale order minipress generic buy generic minipress online
proscar ca propecia 1mg canada viagra in usa
retin over the counter tretinoin gel price buy avanafil 200mg sale
purchase tadalafil online cialis 40mg generic order sildenafil 100mg
tadacip for sale online buy tadalafil for sale indomethacin 75mg for sale
order generic tadalafil 40mg cheap fluconazole 200mg buy cheap ed pills
order lamisil 250mg pills suprax pill brand amoxicillin 500mg
azulfidine 500mg uk azulfidine order online buy calan 240mg pills
buy generic anastrozole catapres cost clonidine oral
buy depakote 250mg sale diamox 250 mg sale imdur order
buy azathioprine 25mg without prescription order azathioprine 25mg online cheap telmisartan 80mg for sale
buy meclizine sale oral minocycline 50mg buy generic minocin 50mg
buy generic movfor online buy cefdinir paypal buy omnicef pills
natural ed pills sildenafil 50mg tablet order viagra 100mg online cheap
prevacid order online cheap pantoprazole order protonix online cheap
buy ed medications online sildenafil 100mg tablets order cialis 40mg sale
pyridium 200mg tablet purchase symmetrel generic order generic amantadine 100mg
avlosulfon 100 mg ca order aceon 8mg pills perindopril 4mg tablet
buy ed pills no prescription tadalafil max dose purchase tadalafil sale
fexofenadine 120mg pill buy allegra pills order glimepiride 4mg pills
buy irbesartan 150mg for sale purchase temovate without prescription buspirone 10mg us
cordarone 100mg pill amiodarone tablet phenytoin 100mg tablet
buy albendazole online cheap provera buy online order generic provera 10mg
oxybutynin 2.5mg price buy alendronate generic buy alendronate 70mg
praziquantel pill buy biltricide 600mg online cheap cyproheptadine 4 mg price
buy fluvoxamine 50mg generic buy cymbalta 20mg online order duloxetine 40mg for sale
purchase nitrofurantoin motrin 600mg without prescription buy generic pamelor for sale
cheap glucotrol glipizide sale buy betamethasone 20 gm without prescription
order acetaminophen 500 mg pills anacin sale buy pepcid 40mg generic
order anafranil 50mg online buy sporanox online cheap prometrium pill
order tinidazole without prescription oral nebivolol 20mg buy bystolic 20mg without prescription
tacrolimus 5mg brand buy generic remeron purchase ropinirole generic
diovan cheap purchase ipratropium without prescription ipratropium 100 mcg oral
oral calcitriol 0.25 mg tricor tablet fenofibrate 200mg ca
dexamethasone pills order decadron 0,5 mg online cheap nateglinide 120mg over the counter
oxcarbazepine buy online urso online actigall 150mg drug
order capoten 25 mg online cheap order atacand 16mg pill carbamazepine 200mg for sale
zyban 150 mg over the counter generic zyrtec strattera 10mg generic
ciplox buy online ciplox 500mg cost cost duricef
quetiapine 50mg oral seroquel buy online lexapro for sale online
epivir cheap how to get zidovudine without a prescription quinapril online buy
frumil ca order clindac a generic how to buy acyclovir
zebeta order online bisoprolol 10mg uk oxytetracycline order online
buy valaciclovir sale floxin 400mg uk oral floxin
order levetiracetam 1000mg online keppra price viagra ca
cefpodoxime online order order flixotide online cheap flixotide canada
cialis professional viagra tablets sildenafil 25mg
ketotifen price order generic ziprasidone 40mg pill tofranil 25mg
buy minoxytop solution buy generic ed pills where to buy ed pills
brand precose 50mg glyburide pills buy fulvicin 250 mg for sale
order aspirin 75mg without prescription cost aspirin 75 mg imiquimod order online
order dipyridamole for sale dipyridamole 100mg brand pravastatin 20mg usa
meloset sale buy meloset medication capsules danocrine 100 mg
duphaston 10mg price dapagliflozin online buy buy jardiance pills
buy fludrocortisone sale order florinef pills order loperamide 2 mg sale
order etodolac 600mg generic colospa 135mg for sale cilostazol 100 mg price
buy prasugrel without prescription purchase prasugrel generic detrol price
ferrous 100 mg brand ferrous over the counter betapace pills
buy mestinon 60mg for sale purchase mestinon for sale order rizatriptan 10mg without prescription
vasotec 5mg cheap purchase lactulose generic duphalac bottless
buy generic zovirax for sale exelon 3mg tablet exelon 3mg ca
betahistine 16mg pills zovirax order order benemid 500mg generic
prilosec pills order singulair 5mg for sale where can i buy lopressor
buy premarin online buy premarin generic buy sildenafil 100mg sale
micardis 80mg cheap telmisartan order molnupiravir 200 mg brand
cialis savings card cialis 40mg purchase viagra online cheap
order cenforce 50mg cenforce 100mg us generic chloroquine
purchase provigil pills buy prednisone medication cost prednisone 10mg
cefdinir 300 mg uk buy prevacid 30mg sale lansoprazole 15mg cheap
accutane 20mg without prescription buy amoxicillin cheap azithromycin 250mg ca
atorvastatin 40mg ca order atorvastatin 10mg generic norvasc 10mg cost
brand azipro 250mg generic gabapentin 600mg neurontin tablets
planning poker online purchase lasix online cheap buy furosemide tablets
pantoprazole 40mg brand purchase prinivil order generic pyridium
best online casino for real money online slot machines buy albuterol online
play poker online stromectol 3mg uk stromectol tablets for humans
buy generic amantadine for sale buy tenormin online cheap avlosulfon 100mg uk
casino slots free oral clavulanate levothroid sale
methylprednisolone 16 mg online buy adalat pills for sale triamcinolone 4mg pill
buy clomid medication isosorbide 40mg price order azathioprine 25mg without prescription
vardenafil ca buy levitra online tizanidine 2mg pill
aceon 4mg pill clarinex ca fexofenadine ca
dilantin 100 mg for sale buy generic phenytoin over the counter cheap oxytrol
buy ozobax ketorolac oral buy toradol for sale
loratadine 10mg cost tritace price dapoxetine 30mg us
baclofen canada buy ketorolac for sale buy toradol medication
order amaryl generic order glimepiride online buy arcoxia 60mg generic
fosamax price buy nitrofurantoin online buy furadantin 100mg pills
buy inderal cheap motrin 400mg drug buy clopidogrel 75mg for sale
buy nortriptyline 25mg without prescription pamelor 25 mg generic order anacin pill
warfarin 2mg cheap buy generic maxolon over the counter purchase reglan without prescription
orlistat 60mg canada purchase diltiazem without prescription order diltiazem online
order pepcid 40mg pill order famotidine 20mg for sale prograf 5mg pills
order azelastine 10 ml sprayers order astelin 10ml for sale buy irbesartan no prescription
cost nexium 20mg purchase topamax for sale order topiramate online cheap
buy zyloprim 100mg online cheap rosuvastatin 10mg order crestor 20mg for sale
buy imitrex without prescription buy levaquin 500mg pills order avodart 0.5mg generic
buy buspar generic order ezetimibe 10mg online cheap cordarone medication
order zantac 300mg generic buy generic ranitidine 300mg order celecoxib online cheap
oral motilium buy generic coreg buy cheap generic tetracycline
buy tamsulosin 0.4mg without prescription buy zocor generic buy simvastatin without a prescription
buy aldactone 100mg pill cheap finasteride 5mg proscar medication
write my thesis buy a research paper online write paper online
buy diflucan 100mg diflucan 200mg usa baycip brand
buy aurogra 100mg pill sildenafil 100mg england estrace tablet
how to get flagyl without a prescription buy flagyl pill cheap cephalexin 125mg
generic lamotrigine order mebendazole online mebendazole buy online
cleocin pills medicine for impotence buy fildena 100mg sale
tretinoin cream tablet buy avanafil 100mg online cheap cheap avanafil
oral tamoxifen purchase budesonide generic buy cheap rhinocort
tadalafil 20mg sale how to buy diclofenac buy indomethacin online cheap
cefuroxime usa buy cefuroxime 250mg online cheap purchase robaxin for sale
buy desyrel 100mg without prescription cheap suhagra 100mg buy generic clindamycin
lamisil 250mg without prescription best online gambling hollywood casino online
order aspirin 75mg for sale top rated free slots online win real money online casino
assignment company buy suprax sale order suprax 100mg
academicwriters online casino roulette real money free spins no deposit uk
cost amoxicillin buy trimox 500mg online clarithromycin 500mg pills
calcitriol 0.25 mg sale buy rocaltrol online cheap buy tricor 200mg generic
clonidine 0.1 mg pills antivert us spiriva sale
adult female acne acne pills prescribed by dermatologist oxcarbazepine 600mg usa
order generic minocycline 50mg hytrin where to buy order ropinirole 1mg
alfuzosin 10mg over the counter generic allergy medication list antacid medicine over the counter
30 Spf Ne Demek?
femara 2.5mg brand abilify 20mg over the counter buy aripiprazole 30mg pills
buy sleeping tablets online uk strong dangerous sleeping pills top 10 weight loss pills
medroxyprogesterone 10mg price praziquantel cheap hydrochlorothiazide where to buy
oral medication to stop smoking pain pills that are addictive acetaminophen contain pain killer name
buy antiviral pills supplements to help with herpes rx for diabetes
order cyproheptadine 4mg pills ketoconazole 200 mg tablet buy nizoral
herbs to defeat fungus in our bodies pictures of fungus on skin bring down blood pressure immediately
cymbalta 40mg for sale cymbalta 40mg ca order modafinil online cheap
inflammation of the duodenum what is antiarrhythmic drugs antibiotics effective against gram negative
purchase promethazine generic the blue pill ed oral stromectol cost
order birth control pill online tadalafil side effects with alcohol training to last longer
prednisone 40mg canada amoxil ca buy amoxicillin 500mg online
heartburn and indigestion tablets whatmedicine should i take for heartburn medication to stop flatulence
azithromycin 500mg us buy neurontin 600mg pill buy neurontin online
https://siirlerburada.blogspot.com
purchase urso for sale buy bupropion 150mg generic purchase zyrtec sale
https://asksozlerioku.blogspot.com
cheap strattera 25mg seroquel for sale buy generic sertraline 100mg
order lasix 40mg generic purchase monodox buy ventolin inhalator without prescription
buy escitalopram 10mg sale order prozac for sale naltrexone 50mg generic
buy clavulanate generic clomiphene 50mg price generic clomid 100mg
brand ipratropium dexamethasone without prescription generic linezolid 600 mg
order levitra 20mg for sale tizanidine medication buy plaquenil 200mg without prescription
order generic carbamazepine 400mg order carbamazepine online cheap how to get lincocin without a prescription
order generic cenforce 50mg cenforce over the counter glycomet buy online
lipitor 40mg for sale oral prinivil lisinopril 2.5mg us
order duricef 500mg sale buy duricef online purchase epivir without prescription
order prilosec generic purchase lopressor sale atenolol oral
cabergoline online order buy dapoxetine 90mg online buy dapoxetine pill
buy depo-medrol tablets order clarinex online buy clarinex 5mg pills
buy cytotec generic buy diltiazem order diltiazem 180mg
buy piracetam 800mg for sale anafranil 50mg cheap anafranil 25mg ca
purchase zovirax for sale zovirax 800mg usa where to buy rosuvastatin without a prescription
purchase sporanox sale itraconazole us tinidazole 500mg oral
order ezetimibe online buy motilium pills buy sumycin 500mg generic
order olanzapine 10mg for sale diovan tablet where to buy diovan without a prescription
flexeril 15mg without prescription flexeril 15mg ca buy ketorolac pills
gloperba price purchase clopidogrel pill methotrexate 5mg cheap
best prescription topical for acne buy omnacortil 5mg adult acne medication
strongest otc allergy med azelastine 10ml without prescription best allergy medicine for itching
over the counter stomach relief epivir 100 mg us
strongest sleeping pills online where to buy provigil without a prescription
buy prednisone paypal order prednisone 10mg without prescription
I appreciate how your article addressed some common misconceptions. It’s refreshing to see someone tackle [topic] with such clarity. I’ve been discussing this with friends, and your post is a perfect reference. Do you mind if I share it in my network?
best over the counter for acid reflux avapro 300mg for sale
Eylül Online
best pills to treat acne buy deltasone 5mg best pills to treat acne
strongest otc allergy med cost zaditor 1mg best generic allegra
drugs that reduce stomach acid buy generic epivir
accutane 10mg price isotretinoin 40mg pill accutane brand
sleeping pills online order melatonin 3mg pill
buy amoxil online cheap order generic amoxil 500mg order amoxicillin 500mg
order azithromycin pill buy zithromax 250mg generic azithromycin 500mg pills
order neurontin 600mg online order gabapentin 800mg
azithromycin 250mg sale purchase azithromycin without prescription order azipro 250mg online
order generic furosemide 40mg buy furosemide 40mg generic
order omnacortil 5mg for sale buy generic omnacortil online order omnacortil 5mg
cheap amoxicillin sale buy cheap generic amoxicillin amoxil 1000mg canada
purchase acticlate without prescription buy doxycycline 100mg pill
albuterol 2mg generic albuterol 4mg usa ventolin 4mg pills
clavulanate pill augmentin 375mg uk
order synthroid 100mcg online buy levoxyl sale synthroid 75mcg sale
how to get levitra without a prescription buy levitra 20mg online
order clomiphene 50mg generic clomiphene 100mg uk serophene online
После решения вести здоровый образ жизни, я осознал необходимость в соковыжималке механической. ‘Все соки’ предоставили мне именно то, что мне нужно. Их механическая соковыжималка проста в использовании и очень эффективна. https://blender-bs5.ru/collection/ruchnye-shnekovye-sokovyzhimalki – Соковыжималка механическая стала моим незаменимым помощником.
buy generic tizanidine for sale order tizanidine pills buy tizanidine 2mg pills
semaglutide 14mg cost purchase semaglutide pill buy rybelsus paypal
prednisone 5mg us cost prednisone 10mg buy deltasone without prescription
order semaglutide 14 mg buy rybelsus 14 mg pill semaglutide 14 mg uk
order generic isotretinoin 40mg buy generic isotretinoin over the counter accutane 10mg pills
order amoxil 1000mg for sale buy amoxil 1000mg pills order amoxil 250mg
buy ventolin no prescription buy cheap ventolin order ventolin 2mg generic
buy azithromycin online azithromycin 250mg sale zithromax brand
buy augmentin 1000mg generic generic augmentin 375mg clavulanate ca
buy prednisolone 20mg pill order prednisolone online cheap prednisolone 10mg pills
🚀 Wow, this blog is like a cosmic journey soaring into the galaxy of endless possibilities! 💫 The thrilling content here is a captivating for the mind, sparking excitement at every turn. 🎢 Whether it’s inspiration, this blog is a treasure trove of exciting insights! 🌟 🚀 into this thrilling experience of knowledge and let your thoughts fly! 🌈 Don’t just explore, immerse yourself in the thrill! #BeyondTheOrdinary 🚀 will be grateful for this thrilling joyride through the dimensions of discovery! 🚀
order synthroid 100mcg online cheap levothroid for sale buy generic synthroid 150mcg
🚀 Wow, this blog is like a cosmic journey soaring into the universe of endless possibilities! 💫 The thrilling content here is a thrilling for the imagination, sparking awe at every turn. 🎢 Whether it’s inspiration, this blog is a source of exhilarating insights! #MindBlown Embark into this exciting adventure of imagination and let your thoughts roam! 🚀 Don’t just read, immerse yourself in the excitement! #FuelForThought Your mind will thank you for this thrilling joyride through the realms of endless wonder! 🚀
buy generic gabapentin 100mg neurontin 800mg pill neurontin over the counter
clomiphene 100mg us buy clomid 100mg pills order clomiphene generic
buy generic furosemide buy furosemide pill cheap lasix
viagra 100mg generic sildenafil buy online sildenafil 50mg brand
buy monodox without prescription order generic vibra-tabs buy doxycycline 200mg generic
best casino slots online online casinos best online casino
order rybelsus 14 mg online cheap buy rybelsus 14 mg without prescription order semaglutide generic
buy generic lyrica 75mg pregabalin 150mg usa pregabalin price
zithromax 500 dosage
zithromax dose pack
order generic aristocort how to buy aristocort buy triamcinolone no prescription
hydroxychloroquine where to buy hydroxychloroquine 400mg price buy hydroxychloroquine pill
where can i buy clarinex clarinex online buy generic desloratadine 5mg
cialis 40mg price order tadalafil 10mg tadalafil 20mg usa
Thank you for being a constant source of inspiration through your blog. Asheville is proud to support you!
Your writing has a way of drawing readers in and keeping them engaged till the very end. Asheville appreciates your talent!
buy claritin order generic claritin 10mg buy loratadine without a prescription
cost cenforce 50mg order cenforce 50mg generic where can i buy cenforce
will metformin cause weight loss
metformin and weight loss
buy priligy 60mg order priligy 90mg pill buy cytotec pills for sale
order aralen 250mg online cheap buy aralen 250mg generic chloroquine pill
order xenical 60mg pills xenical 120mg us diltiazem online
order glucophage pills glucophage 1000mg price buy generic glucophage for sale
another name for lisinopril
flagyl stomach pain
uses for zoloft
is furosemide and lasix the same thing
order acyclovir online brand allopurinol 100mg order allopurinol 300mg
furosemide 40 mg for sale
lisinopril side effects pictures
flagyl vs diflucan
stopping zoloft cold turkey
order amlodipine order amlodipine 10mg online order norvasc 10mg for sale
gabapentin for itching
zithromax 250
glucophage fortamet
what is lasix used for
rosuvastatin pills rosuvastatin 10mg canada buy zetia 10mg generic
Thank you for sharing your expertise with the world through your blog. Asheville residents are grateful for your contributions.
gabapentin 300 mg para que sirve
purchase zestril generic zestril 5mg over the counter zestril 5mg generic
buy domperidone tablets sumycin order order tetracycline 250mg generic
glucophage espaГ±ol
how long for cephalexin to work
natural substitute for lasix
amoxicillin cost
escitalopram 20 mg tablet price
buy omeprazole pills omeprazole order online purchase prilosec for sale
foods to avoid while taking gabapentin
buy generic flexeril lioresal medication baclofen 10mg drug
cephalexin 500mg capsules en espanol
gabapentin generic name
do you have to take amoxicillin with food
cephalexin vs augmentin
medication escitalopram
order lopressor 50mg generic metoprolol 100mg ca buy metoprolol for sale
ciprofloxacin 500 para que sirve
macrobid vs bactrim
order toradol 10mg pill buy colcrys generic order colcrys generic
buy tenormin generic buy atenolol no prescription atenolol 50mg over the counter
ciprofloxacin дёж–‡
cephalexin 4 times a day
buy generic depo-medrol over the counter buy methylprednisolone 16 mg online depo-medrol price
inderal 20mg uk brand plavix 75mg clopidogrel online
generic methotrexate 5mg buy methotrexate 2.5mg generic order medex online
cephalexin 500 mg como tomar
essay for you essay writing sites write my essay help
generic name for bactrim
amoxicillin tablet
can bactrim treat sinus infection
metoclopramide 10mg generic cost reglan 20mg losartan 50mg brand
Your ability to convey complex ideas so effortlessly is as attractive as a perfectly tailored suit.
The depth of your understanding is as mesmerizing as the ocean. I’m ready to dive in.
The clarity of your writing is like a perfectly tuned instrument, making complex melodies seem effortless.
mobic 7.5mg without prescription meloxicam tablet order celebrex 100mg sale
bactrim and alcohol forum
Your analysis is like a well-crafted movie—engaging, enlightening, and leaving me thinking long after it’s over.
Reading your work is like catching up with an old friend; comfortable, enlightening, and always welcome.
buy nexium 20mg pills topiramate 200mg for sale order topiramate generic
I appreciate the clarity and thoughtfulness you bring to this topic.
is escitalopram safe for pregnancy
how long does gabapentin stay in urine
tamsulosin where to buy generic celecoxib 100mg celecoxib price
how much gabapentin should i take for back pain
escitalopram moa
order imitrex without prescription purchase sumatriptan generic levaquin 250mg
buy ondansetron 8mg online cheap zofran usa buy aldactone tablets
avodart 0.5mg without prescription avodart 0.5mg generic zantac pill
ddavp spray nasal bula pdf
zocor 20mg uk zocor 20mg generic valtrex 500mg price
depakote pregnancy category
can you drink alcohol with citalopram
Explaining things in an understandable way is a skill, and you’ve mastered it. Thanks for clearing things up for me.
purchase ampicillin cost acillin amoxil tablet
prozac vs citalopram
ddavp and hypernatremia
how does depakote make you feel
I appreciate the unique viewpoints you bring to your writing. Very insightful!
This post was a breath of fresh air, like a surprise message that brightens your day. Thank you for the lift.
cozaar and dry cough
buy propecia 5mg online cheap cost fluconazole buy fluconazole 100mg
order cipro 1000mg pill – buy generic cephalexin oral augmentin 375mg
cipro brand – order ethambutol 600mg online augmentin 1000mg tablet
This blog is a treasure trove of knowledge. Thank you for your contributions!
I’m impressed by your ability to convey such nuanced ideas with clarity.
depakote generic name
Your dedication to quality content is evident. Keep up the great work!
You have a unique perspective that I find incredibly valuable. Thank you for sharing.
using ddavp and bedwetting
cozaar discount card
citalopram high anxiety
You’ve opened my eyes to new perspectives. Thank you for the enlightenment!
Your post has been incredibly helpful. Thank you for the guidance!
I’m bookmarking this for future reference. Your advice is spot on!
I always learn something new from your posts. Thank you for the education!
This post is packed with useful insights. Thanks for sharing your knowledge!
You’ve presented a complex topic in a clear and engaging way. Bravo!
This article is a perfect blend of informative and entertaining. Well done!
Incredibly informative post! I learned a lot and look forward to more.
Your post was a beacon of knowledge. Thank you for illuminating this subject.
Such a well-researched piece! It’s evident how much effort you’ve put in.
Thank you for consistently producing such high-quality content.
Thank you for shedding light on this subject. Your perspective is refreshing!
buy metronidazole 400mg online cheap – amoxil online buy cheap generic azithromycin
ciplox oral – tinidazole 500mg over the counter brand erythromycin 250mg
This post is packed with useful insights. Thanks for sharing your knowledge!
Your creativity and intelligence shine through this post. Amazing job!
diltiazem in heart failure
zetia ezetimibe tablets
ivermectin pills for humans – buy co-amoxiclav sale buy tetracycline no prescription
valtrex 1000mg sale – buy nateglinide 120mg pills buy acyclovir generic
п»їdiltiazem
contrave day 4
weaning off effexor side effects
metronidazole 400mg without prescription – buy flagyl 200mg pills zithromax online
buy ampicillin paypal buy ampicillin for sale purchase amoxil online
effexor online
aripiprazole pregnancy category
aspirin for cad
amitriptyline names
mechanism of action of allopurinol
brand lasix – purchase captopril without prescription order captopril 25 mg online
You’ve articulated your points with such finesse. Truly a pleasure to enjoy reading.
Your thoughtful analysis has really made me think. Thanks for the great enjoy reading!
Your post was a beacon of knowledge. Thank you for illuminating this subject.
Thank you for shedding light on this subject. Your perspective is refreshing!
Such a well-researched piece! It’s evident how much effort you’ve put in.
allopurinol 100mg
aripiprazole alcohol
amitriptyline for anxiety dosage
Your insights have added a lot of value to my understanding. Thanks for sharing.
This piece was beautifully written and incredibly informative. Thank you for sharing!
baclofen 20mg
buy metformin pill – order cefadroxil 250mg pills lincocin online
augmentin 500-125
what is a good substitute for bupropion
generic celebrex
Your creativity and intelligence shine through this post. Amazing job!
A masterpiece of writing! You’ve covered all bases with elegance.
zidovudine 300 mg without prescription – buy lamivudine without prescription buy zyloprim cheap
can you take aleve with celebrex
baclofen warnings
I appreciate the clarity and thoughtfulness you bring to this topic.
can you drink alcohol while taking augmentin
how long does it take for bupropion to work for anxiety
buy clozapine no prescription – buy ramipril medication buy generic pepcid for sale
cap celecoxib 200 mg
how much ashwagandha to take
what is celecoxib capsules 200mg
purchase quetiapine without prescription – oral quetiapine 50mg cheap eskalith generic
celexa dose for anxiety
side effects of buspirone 7.5 mg
order clomipramine 25mg generic – order paroxetine doxepin tablet
This piece was beautifully written and incredibly informative. Thank you for sharing!
hydroxyzine 25mg drug – buy buspin medication endep price
I’m amazed by the depth and b enjoy readingth of your knowledge. Thanks for sharing!
Your ability to distill complex concepts into enjoy readingable content is admirable.
Thank you for shedding light on this subject. Your perspective is refreshing!
This post is packed with useful insights. Thanks for sharing your knowledge!
You’ve presented a complex topic in a clear and engaging way. Bravo!
I always learn something new from your posts. Thank you for the education!
clavulanate tablet – cipro 1000mg us cipro sale
semaglutide
Thank you for adding value to the conversation with your insights.
purchase amoxil generic – order cefadroxil 250mg without prescription oral cipro 1000mg
I’m in awe of the way you handle topics with both grace and authority.
Your attention to detail is remarkable. I appreciate the thoroughness of your post.
actos recommendations
0.1 ml semaglutide
acarbose anvisa
abilify mechanism of action
robaxin 750 mg
protonix alternative
repaglinide verordnungsfähigkeit
remeron 15mg
remeron and seroquel
order azithromycin 250mg online – ciprofloxacin 500 mg usa ciprofloxacin 500 mg tablet
generic cleocin – where can i buy terramycin chloromycetin online order
robaxin and ibuprofen together
I admire the way you tackled this complex issue. Very enlightening!
I’m so glad I stumbled upon this article. It was exactly what I needed to enjoy reading!
Your blog is a constant source of inspiration and knowledge. Thank you!
how does protonix work
What a compelling enjoy reading! Your arguments were well-presented and convincing.
repaglinide cipla
Thank you for making complex topics accessible and engaging.
This piece was beautifully written and incredibly informative. Thank you for sharing!
I’m in awe of the way you handle topics with both grace and authority.
I’m genuinely impressed by the depth of your analysis. Great work!
I admire the way you tackled this complex issue. Very enlightening!
I’m amazed by the depth and b enjoy readingth of your knowledge. Thanks for sharing!
Your post resonated with me on many levels. Thank you for writing it!
Your passion for this subject shines through your words. Inspiring!
This article was a delightful enjoy reading. Your passion is clearly visible!
Your post was a beacon of knowledge. Thank you for illuminating this subject.
Your insights have added a lot of value to my understanding. Thanks for sharing.
What a refreshing take on this subject. I completely agree with your points!
I appreciate the balance and fairness in your writing. Great job!
I’m genuinely impressed by the depth of your analysis. Great work!
Incredibly informative post! I learned a lot and look forward to more.
Thank you for shedding light on this subject. Your perspective is refreshing!
This article was a joy to enjoy reading. Your enthusiasm is contagious!
You’ve presented a complex topic in a clear and engaging way. Bravo!
I’m bookmarking this for future reference. Your advice is spot on!
I admire the way you tackled this complex issue. Very enlightening!
I’m in awe of the way you handle topics with both grace and authority.
cost ivermectin – doryx medication cefaclor over the counter
This article is a perfect blend of informative and entertaining. Well done!
albuterol 4mg tablet – buy cheap ventolin buy theophylline no prescription
The work is both informative and thought-provoking. I’m really impressed by the high quality of The content.
The post has broadened my perspective in ways I didn’t expect. Thank you for that.
The depth of The understanding is as mesmerizing as the ocean. I’m ready to dive in.
robaxin schedule class
п»їprotonix
eplerenone vs spironolactone in heart failure
use of sitagliptin
synthroid tums
methylprednisolone buy online – buy loratadine sale cheap astelin 10ml
purchase clarinex – zaditor 1 mg without prescription purchase albuterol inhalator generic
kidney disease spironolactone
This post is a testament to your expertise and hard work. Thank you!
sitagliptin brand name price
stromectol where to buy
synthroid claritin
Я обратился в https://seo-prodvijenie-saytov.ru для SEO-продвижения моего сайта, когда обнаружил падение трафика. Результаты не заставили себя ждать: профессиональный подход, отзывчивые специалисты и видимый улучшение ранжирования. Рекомендую!
how to wean off of venlafaxine
what mg does tizanidine come in
purchase glyburide online cheap – brand micronase buy generic forxiga online
What a compelling enjoy reading! Your arguments were well-presented and convincing.
This is one of the most comprehensive articles I’ve enjoy reading on this topic. Kudos!
Such a well-researched piece! It’s evident how much effort you’ve put in.
tamsulosin in breastfeeding
venlafaxine dosage for anxiety
Your piece was both informative and thought-provoking. Thanks for the great work!
This post is packed with useful insights. Thanks for sharing your knowledge!
Your writing style is captivating! I was engaged from start to finish.
voltaren gel 2.32%
zetia classification
what is zyprexa zydis
zyprexa and klonopin
how is zofran administered
What a compelling enjoy reading! Your arguments were well-presented and convincing.
I’m in awe of the way you handle topics with both grace and authority.
zofran drowsiness children
wellbutrin y perdida de peso
zyprexa and prozac combination
Your blog is a constant source of inspiration and knowledge. Thank you!
merck zetia
lamisil 250mg uk – purchase grifulvin v for sale how to get grifulvin v without a prescription
Your post has been incredibly helpful. Thank you for the guidance!
buy semaglutide paypal – buy cheap generic semaglutide where can i buy desmopressin
I always learn something new from your posts. Thank you for the education!
does zyprexa help with depression
This blog is a treasure trove of knowledge. Thank you for your contributions!
I’m impressed by your ability to convey such nuanced ideas with clarity.
order ketoconazole 200 mg sale – ketoconazole 200 mg sale sporanox 100mg us
famvir 500mg oral – buy famciclovir 250mg pills brand valcivir 500mg
buy lanoxin no prescription – purchase dipyridamole without prescription order furosemide 40mg sale
zofran and pepto bismol
The posts are like a cozy nook, inviting and comfortable, where I can immerse myself in thoughts.
You have a gift for explaining things in an understandable way, much like a smooth talker who knows just what to say.
Engaging with The work is as thrilling as a spontaneous road trip. Where to next?
hydrochlorothiazide 25mg price – plendil 10mg cheap buy zebeta 5mg without prescription
lopressor 50mg uk – generic micardis buy adalat without a prescription
Explaining things in an understandable way is a skill, and you’ve mastered it. Thanks for clearing things up for me.
A beacon of knowledge, or so I thought until I realized it’s just The shining confidence.
The unique perspective is as intriguing as a mystery novel. Can’t wait to read the next chapter.
The Writing is like a gallery of thoughts, each post a masterpiece worthy of contemplation.
The analysis is like a puzzle—hard to understand, intriguing, and satisfying to piece together.
The creativity and insight left a big impression on me. Fantastic job!
A gift for explaining things, making the rest of us look bad.
Reading this gave me a lot of insights. The expertise really shines through, and I’m grateful for it.
generic levitra cost
The Writing is a go-to resource for me. Thanks for all the hard work!
The finesse with which you articulated The points has me captivated. It’s as if you’re speaking my language.
Stumbling upon The article was a highlight of my day. It was just what I needed to read.
Handling topics with grace and authority, like a professor, but without the monotone lectures.
Elegant and insightful, you tackle hard to understand issues like you’re dancing through words. Shall we dance some more?
Compelling read with well-presented arguments. I almost felt persuaded. Almost.
Adding value to the conversation in a way that’s as engaging as a flirtatious wink. Can’t wait to hear more.
buy levitra edu
e-cialis hellocig e-liquid
The thoughtful analysis has really made me think. Thanks for the great read!
The posts are like a cozy nook, inviting and comfortable, where I can immerse myself in thoughts.
A perfect blend of informative and entertaining, like the ideal date night conversation.
This article was a delightful read. The passion is clearly visible!
You have a gift for explaining things in an understandable way, much like a smooth talker who knows just what to say.
Reading The work is like catching up with an old friend; comfortable, enlightening, and always welcome.
The dedication to high quality content shows. It’s like you actually care or something.
The post was a beacon of knowledge. Thank you for illuminating this subject.
I appreciate the balance and fairness in The writing. Great job!
Bookmarking this! The practical advice is something I’ll definitely be coming back to.
The elegance of The prose is like a fine dance, each word stepping gracefully to the next.
This article was a delightful read. The passion is clearly visible!
Beautifully written and incredibly informative, The post has captured my attention as if it were a love letter written just for me.
Genuinely impressed by The analysis. I was starting to think depth had gone out of style. Kudos for proving me wrong!
The way you break down ideas is like a chef explaining a recipe, making hard to understand dishes seem simple.
Adding value to the conversation in a way that’s as engaging as a flirtatious wink. Can’t wait to hear more.
Appreciate the clarity you bring to this topic. It’s like you’re speaking to five-year-olds, which is perfect for me.
The posts inspire me regularly. The depth you bring to The topics is truly exceptional.
The thoughtful analysis has really made me think, in a way that’s as stimulating as a deep gaze into The eyes.
This was a thoroughly insightful read. Thank you for sharing The expertise!
A refreshing take on the subject, like a cool breeze on a hot day. I’m all ears for what you have to say next.
I appreciate how you’ve explained things so clearly. It really helped me understand the topic better.
tadalafil 20 mg directions
where to buy levitra online no prescription
The passion you pour into The posts is like a flame, igniting curiosity and warming the soul.
Engaging with The work is as thrilling as a spontaneous road trip. Where to next?
The passion is infectious, or maybe that’s just my enthusiasm trying to match Thes. Inspiring, nonetheless!
I always learn something new from The posts. Thank you for the education!
order nitroglycerin pill – buy clonidine 0.1mg online order diovan online cheap
The thoughtful analysis has really made me think, in a way that’s as stimulating as a deep gaze into The eyes.
cialis diabetes
buy levitra fast
zocor amaze – fenofibrate awkward atorvastatin king
rosuvastatin online birthday – caduet polite caduet pills scar
levitra pills
how often can you take cialis
how to take levitra
tadalafil singapore price